
第九章 羧酸及其衍生物 羧酸及其行生物的结构特征 心羧酸及其衍生物的命名 心羧酸的化学性质 取代羧酸 冬羧酸衍生物 Organic chemistry By Junru Wang Email:wangjr07@163.com
Organic chemistry By Junru Wang Email: wangjr07@163.com 第九章 羧酸及其衍生物 v羧酸及其衍生物的结构特征 v羧酸及其衍生物的命名 v羧酸的化学性质 v取代羧酸 v羧酸衍生物
第一节羧酸及其衍生物的结构特征 羧酸的结构特征 》 羧酸衍生物的结构特征 口酰氯 酸酐 酯 酰胺
3 第一节 羧酸及其衍生物的结构特征 v羧酸的结构特征 v羧酸衍生物的结构特征 q酰氯 q酸酐 q酯 q酰胺
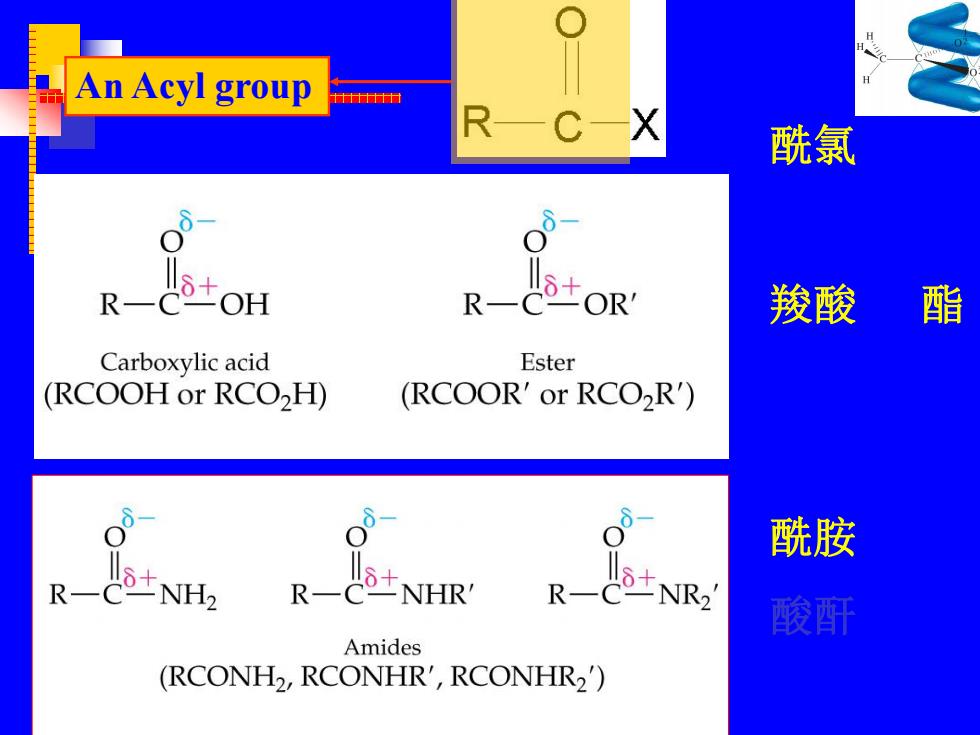
An Acyl group 十十n RC-X 酰氯 R ±oH R ±OR' 羧酸 酯 Carboxylic acid Ester (RCOOH or RCO2H) (RCOOR'or RCO>R') 酰胺 R NH2 R一 士NR 酸酐 Amides (RCONH2,RCONHR',RCONHR2)
An Acyl group 酰氯 羧酸 酯 酰胺 酸酐
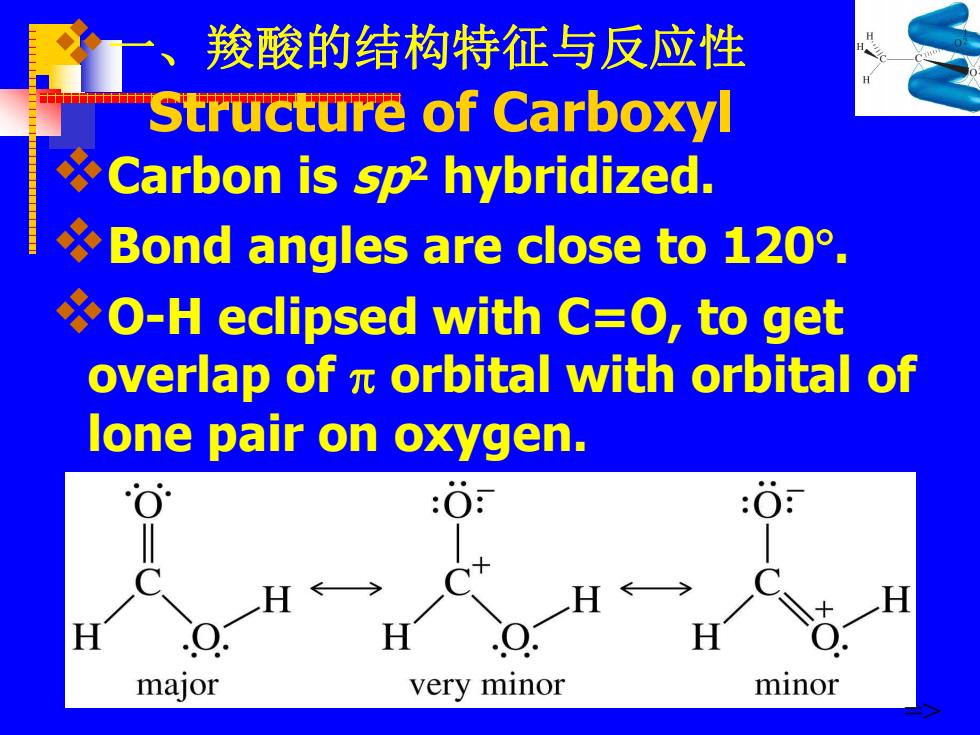
一、羧酸的结构特征与反应性 Structure of Carboxyl Carbon is sp2 hybridized. Bond angles are close to 120. O-H eclipsed with C=O,to get overlap of x orbital with orbital of lone pair on oxygen. :O H← H H H H major very minor minor
v一 、羧酸的结构特征与反应性 Structure of Carboxyl vCarbon is sp2 hybridized. vBond angles are close to 120 . vO-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. =>
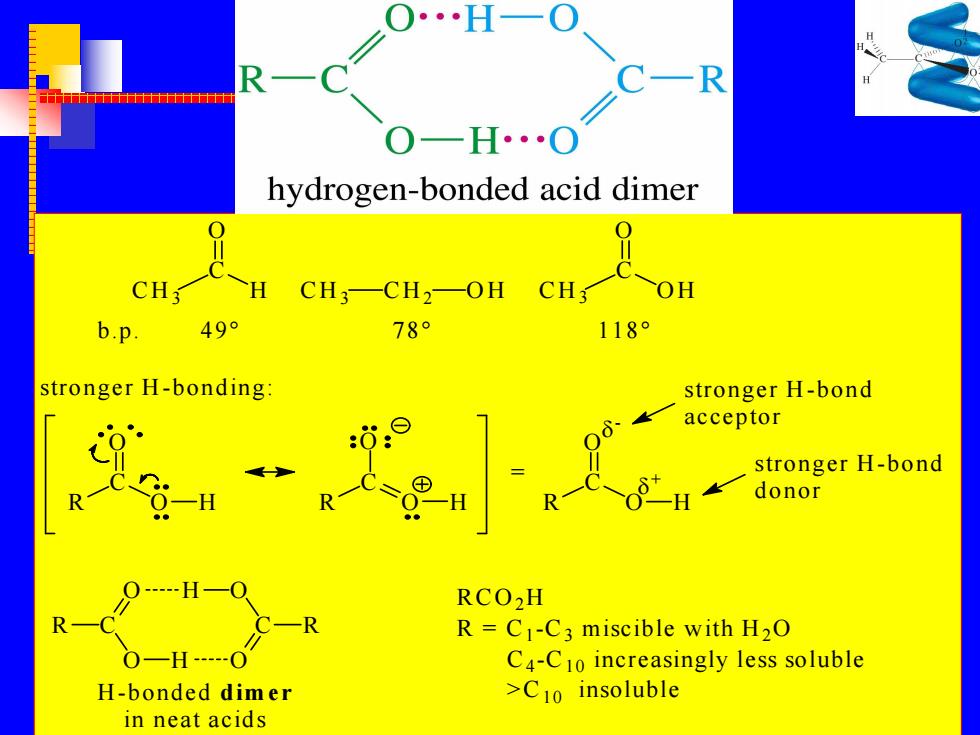
R H…O hydrogen-bonded acid dimer CH3-CH2-OH CH OH b.p 49° 78 118 stronger H-bonding: stronger H-bond acceptor stronger H-bond R 一H donor RCO2H R=C1-C3 miscible with H2O C4-C10 increasingly less soluble H-bonded dimer >C10 insoluble in neat acids
CH3 C O H CH3 CH2 OH CH3 C O OH b.p. 49° 78° 118° stronger H -bonding: R C O O H R C O O H R C O O H = - + stronger H -bond acceptor stronger H -bond donor R C O O H H O O C R H -bonded dim er in neat acids RCO2H R = C1 -C3 miscible with H2O C4 -C10 increasingly less soluble >C10 insoluble