
Uses of Alkyl Halides Industrial and household cleaners. ·Anesthetics: CHCla used originally as general anesthetic but it is toxic and carcinogenic. CFaCHCIBr is a mixed halide sold as HalothaneR Freons are used as refrigerants and foaming agents. 。·Freons can harm the ozone layer so they have been replaced by low-boiling hydrocarbons or carbon dioxide. Pesticides such as DDT are extremely toxic to insects but not as toxic to mammals. Haloalkanes can not be destroyed by bacteria so they accumulate in the soil to a level which can be toxic to mammals,especially,humans. Chapter6 11
Chapter 6 11 Uses of Alkyl Halides • Industrial and household cleaners. • Anesthetics: • CHCl3 used originally as general anesthetic but it is toxic and carcinogenic. • CF3CHClBr is a mixed halide sold as Halothane® • Freons are used as refrigerants and foaming agents. • Freons can harm the ozone layer so they have been replaced by low-boiling hydrocarbons or carbon dioxide. • Pesticides such as DDT are extremely toxic to insects but not as toxic to mammals. • Haloalkanes can not be destroyed by bacteria so they accumulate in the soil to a level which can be toxic to mammals, especially, humans
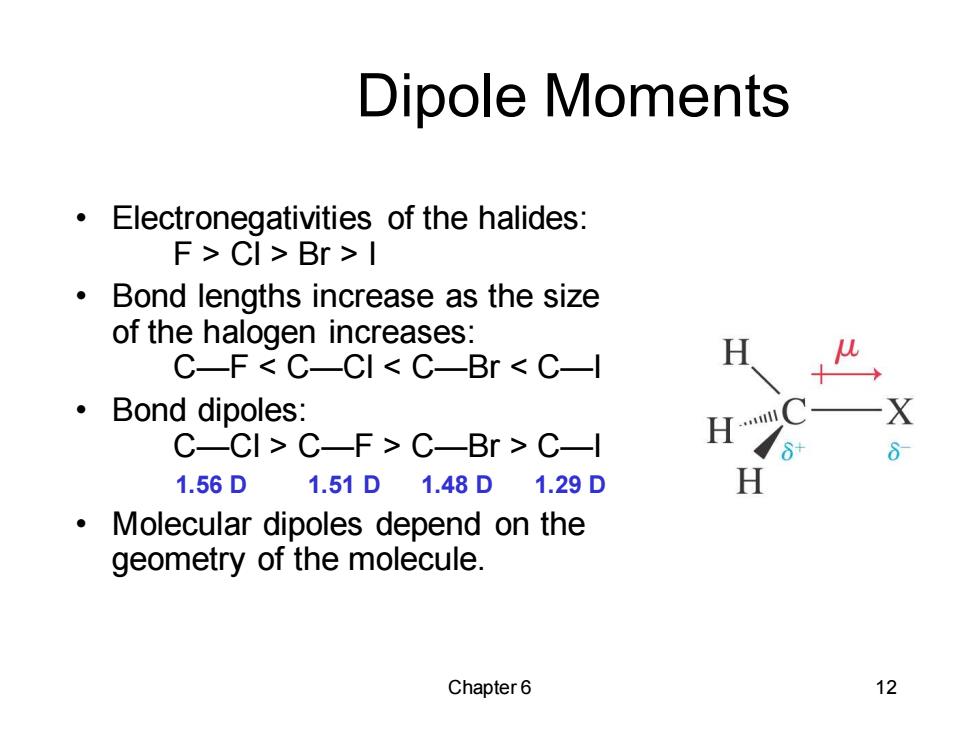
Dipole Moments Electronegativities of the halides: F>CI>Br>I ● Bond lengths increase as the size of the halogen increases: C-F C-CI C-Br C-l ·Bond dipoles: C-CI>C-F>C-Br C-l 1.56D1.51D1.48D1.29D Molecular dipoles depend on the geometry of the molecule. Chapter6 12
Chapter 6 12 Dipole Moments • Electronegativities of the halides: F > Cl > Br > I • Bond lengths increase as the size of the halogen increases: C—F < C—Cl < C—Br < C—I • Bond dipoles: C—Cl > C—F > C—Br > C—I 1.56 D 1.51 D 1.48 D 1.29 D • Molecular dipoles depend on the geometry of the molecule
Boiling Points Greater intermolecular forces,higher b.p. dipole-dipole attractions not significantly different for different halides London forces greater for larger atoms Greater mass,higher b.p. Spherical shape decreases b.p. (CH3)3CBr CH3(CH2)3Br 73C 102C Chapter6 13
Chapter 6 13 Boiling Points • Greater intermolecular forces, higher b.p. • dipole-dipole attractions not significantly different for different halides • London forces greater for larger atoms • Greater mass, higher b.p. • Spherical shape decreases b.p. (CH3 )3CBr CH3 (CH2 )3Br 73C 102C

Densities Alkyl fluorides and chlorides less dense than water. Alkyl dichlorides,bromides,and iodides more dense than water. Chapter 6 14
Chapter 6 14 Densities • Alkyl fluorides and chlorides less dense than water. • Alkyl dichlorides, bromides, and iodides more dense than water

Preparation of Alkyl Halides Free radical halogenation(Chapter 4) Chlorination produces a mixtures of products.This reaction is not a good lab synthesis,except in alkanes where all hydrogens are equivalent. Bromination is highly selective. Free radical allylic halogenation Halogen is placed on a carbon directly attached to the double bond (allylic). Chapter6 15
Chapter 6 15 Preparation of Alkyl Halides • Free radical halogenation (Chapter 4) • Chlorination produces a mixtures of products. This reaction is not a good lab synthesis, except in alkanes where all hydrogens are equivalent. • Bromination is highly selective. • Free radical allylic halogenation • Halogen is placed on a carbon directly attached to the double bond (allylic)