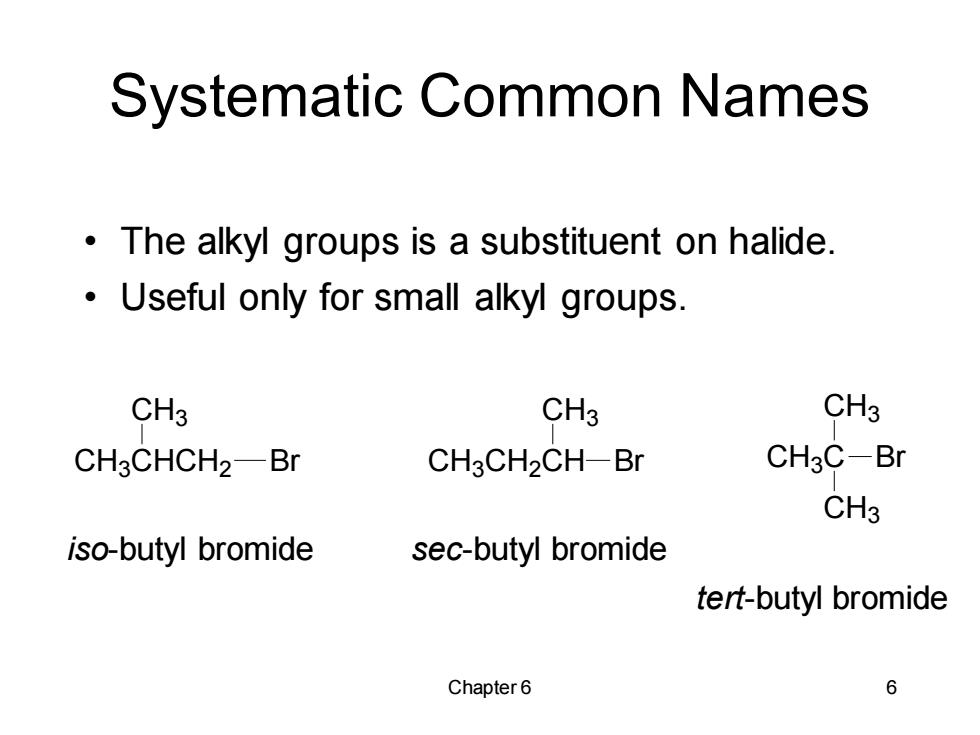
Systematic Common Names The alkyl groups is a substituent on halide. Useful only for small alkyl groups. CH3 CH3 CH3 CH3CHCH2-Br CH3CH2CH-Br CH3C-Br CH3 iso-butyl bromide sec-butyl bromide tert-butyl bromide Chapter 6 6
Chapter 6 6 Systematic Common Names • The alkyl groups is a substituent on halide. • Useful only for small alkyl groups. CH3CHCH2 CH3 Br CH3CH2CH CH3 Br CH3C CH3 Br CH3 iso-butyl bromide sec-butyl bromide tert-butyl bromide

Common Names of Halides 。 CH2X2 called methylene halide. CHX3 is a haloform. 。 CX is carbon tetrahalide. Common halogenated solvents: CH2Cl2 is methylene chloride CHCla is chloroform CCl,is carbon tetrachloride. Chapter6 7
Chapter 6 7 Common Names of Halides • CH2X2 called methylene halide. • CHX3 is a haloform. • CX4 is carbon tetrahalide. • Common halogenated solvents: CH2Cl2 is methylene chloride CHCl3 is chloroform CCl4 is carbon tetrachloride

Alkyl Halides Classification Methyl halides:halide is attached to a methyl group. Primary alkyl halide:carbon to which halogen is bonded is attached to only one other carbon. Secondary alkyl halide carbon to which halogen is bonded is attached to two other carbons. 。 Tertiary alkyl halide carbon to which halogen is bonded is attached to three other carbon. Chapter 6 8
Chapter 6 8 Alkyl Halides Classification • Methyl halides: halide is attached to a methyl group. • Primary alkyl halide: carbon to which halogen is bonded is attached to only one other carbon. • Secondary alkyl halide : carbon to which halogen is bonded is attached to two other carbons. • Tertiary alkyl halide : carbon to which halogen is bonded is attached to three other carbon
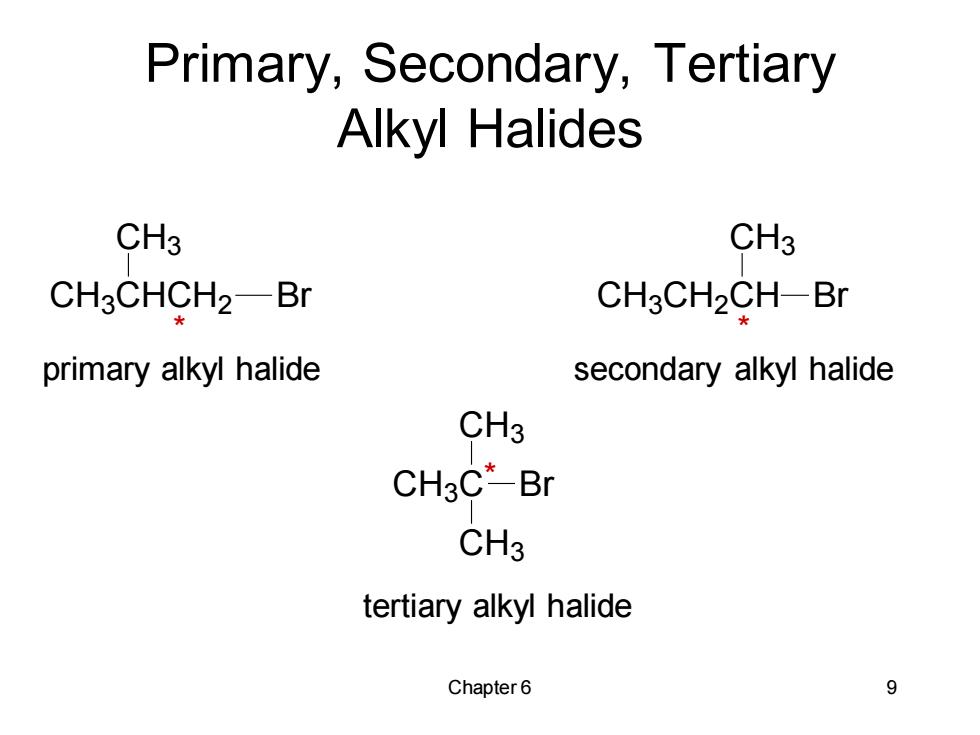
Primary,Secondary,Tertiary Alkyl Halides CH3 CH3 CH3CHCH2-Br CH3CH2CH-Br primary alkyl halide secondary alkyl halide CH3 CH3C*-Br CH3 tertiary alkyl halide Chapter 6 9
Chapter 6 9 primary alkyl halide secondary alkyl halide tertiary alkyl halide Primary, Secondary, Tertiary Alkyl Halides CH3 CHCH2 CH3 Br CH3 CH2 CH CH3 Br CH3 C CH3 Br CH3 * * *

Types of Dihalides 。Geminal dihalide: Br two halogen atoms CH3-CH-Br are bonded to the same carbon. geminal dihalide ·Vicinal dihalide: two halogen atoms Br-CH2CH2-Br are bonded to adjacent carbons. vicinal dihalide Chapter 6 10
Chapter 6 10 Types of Dihalides • Geminal dihalide: two halogen atoms are bonded to the same carbon. • Vicinal dihalide: two halogen atoms are bonded to adjacent carbons. CH3 CH Br Br Br CH2 CH2 Br geminal dihalide vicinal dihalide