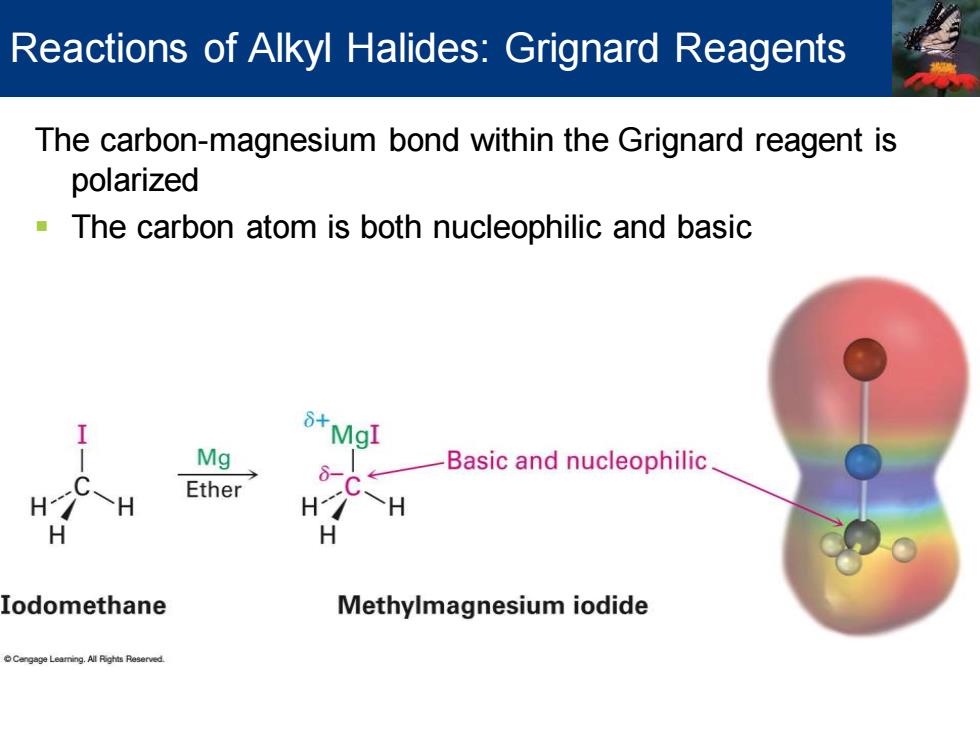
Reactions of Alkyl Halides:Grignard Reagents The carbon-magnesium bond within the Grignard reagent is polarized The carbon atom is both nucleophilic and basic 8+MgI Mg 8-C Basic and nucleophilic Ether H H Iodomethane Methylmagnesium iodide
The carbon-magnesium bond within the Grignard reagent is polarized ▪ The carbon atom is both nucleophilic and basic Reactions of Alkyl Halides: Grignard Reagents

Reactions of Alkyl Halides:Grignard Reagents Grignard Reagents are magnesium salts,R3C-+MgX,of a carbon acid,R3C-H They react with weak acids such as H2O,ROH,RCO2H,and RNH2 to abstract a proton and yield hydrocarbons Since hydrocarbons are weak acids,with pKa's in the range of 44 to 60,carbon anions are very strong bases CH3CH2CH2CH2CH2CH2Br Mg→ H20 Ether CH3CH2CH2CH2CH2CH2MgBr CH3CH2CH2CH2CH2CH3 1-Bromohexane 1-Hexylmagnesium bromide Hexane Cengage Learing.All Rights Resorved. Alkyl halide Grignard Reagent Hydrocarbon They have no role in biochemistry of living organisms They are useful carbon-based nucleophiles in many laboratory reactions They act as models for more complex carbon-based nucleophiles that are important in biological chemistry
Grignard Reagents are magnesium salts, R3C- +MgX, of a carbon acid, R3C-H ▪ They react with weak acids such as H2O, ROH, RCO2H, and RNH2 to abstract a proton and yield hydrocarbons ▪ Since hydrocarbons are weak acids, with pKa ’s in the range of 44 to 60, carbon anions are very strong bases ▪ They have no role in biochemistry of living organisms ▪ They are useful carbon-based nucleophiles in many laboratory reactions ▪ They act as models for more complex carbon-based nucleophiles that are important in biological chemistry Alkyl halide Grignard Reagent Hydrocarbon Reactions of Alkyl Halides: Grignard Reagents

12-5 Organometallic Coupling Reactions Many organometallic compounds that are similar to Grignard reagents Alkyllithium reagents,RLi,can be prepared by the reaction of an alkyl halide with lithium metal Alkyllithiums are both nucleophiles and strong bases with similar chemistry to alkylmagnesium halides Basic and nucleophilic 2Li CH3CH2CH2CH2Br Pentane CH3CH2CH2CH2Li LiBr 1-Bromobutane Butyllithium Cengge Leaming All Rigts Raserd
Many organometallic compounds that are similar to Grignard reagents • Alkyllithium reagents, RLi, can be prepared by the reaction of an alkyl halide with lithium metal • Alkyllithiums are both nucleophiles and strong bases with similar chemistry to alkylmagnesium halides 12-5 Organometallic Coupling Reactions
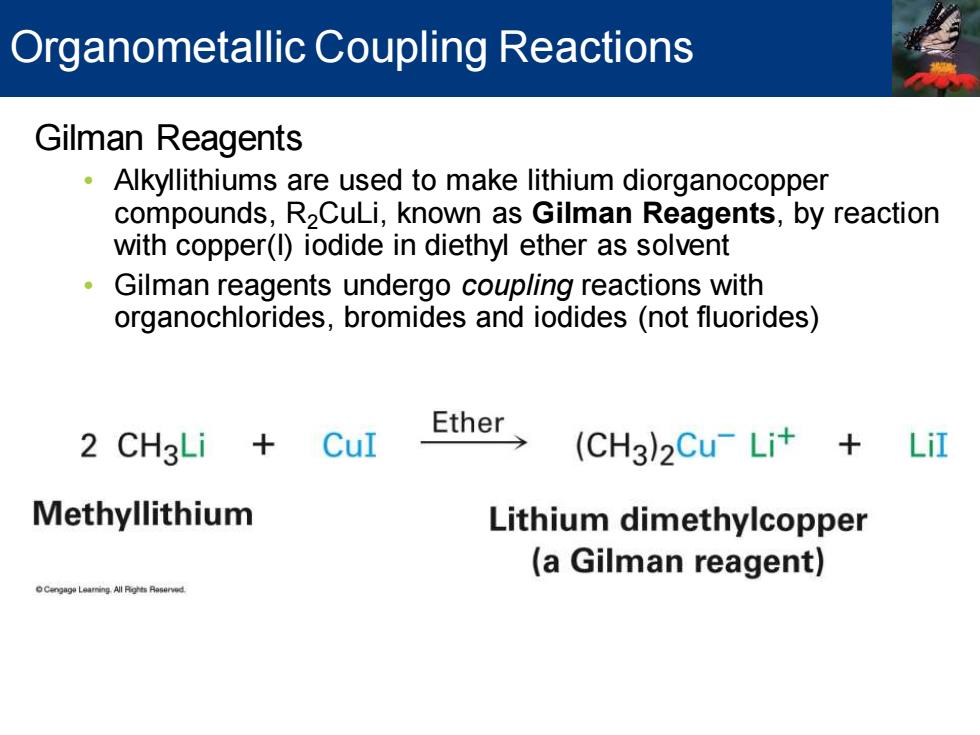
Organometallic Coupling Reactions Gilman Reagents Alkyllithiums are used to make lithium diorganocopper compounds,R2CuLi,known as Gilman Reagents,by reaction with copper(l)iodide in diethyl ether as solvent 。 Gilman reagents undergo coupling reactions with organochlorides,bromides and iodides(not fluorides) 2 CH3Li CuI Ether> (CH3)2Cu-Lit LiI Methyllithium Lithium dimethylcopper (a Gilman reagent) Cengag Leaming All Pights Raserved
Gilman Reagents • Alkyllithiums are used to make lithium diorganocopper compounds, R2CuLi, known as Gilman Reagents, by reaction with copper(I) iodide in diethyl ether as solvent • Gilman reagents undergo coupling reactions with organochlorides, bromides and iodides (not fluorides) Organometallic Coupling Reactions
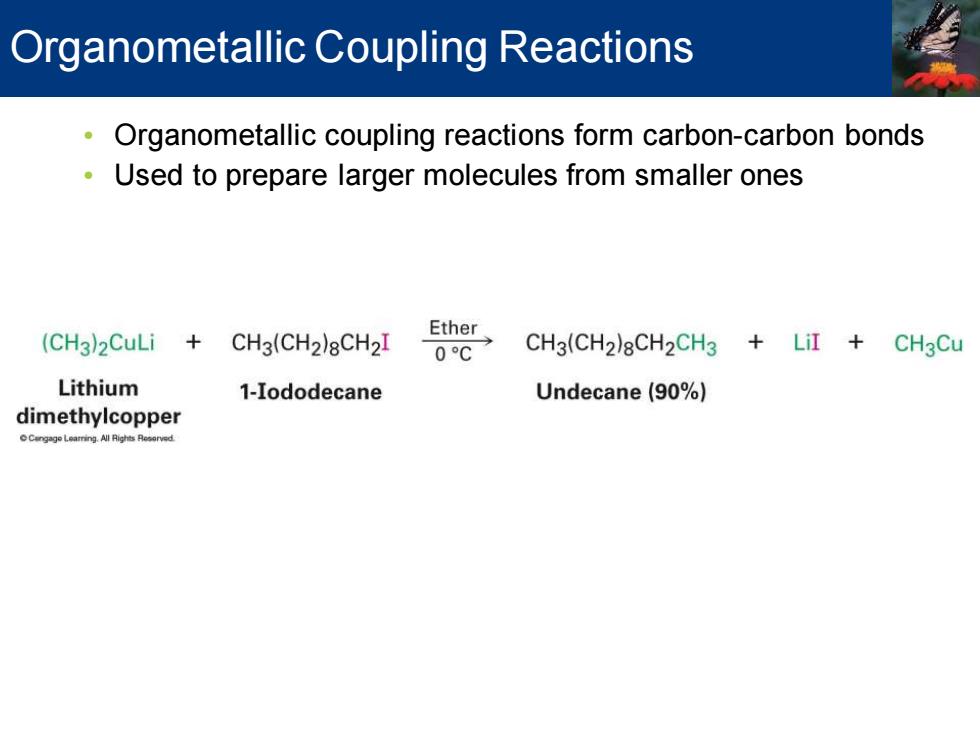
Organometallic Coupling Reactions Organometallic coupling reactions form carbon-carbon bonds Used to prepare larger molecules from smaller ones (CH3)2CuLi+CH3(CH2)8CH2I Ether 0C CH3(CH2)8CH2CH3 LiI CH3Cu Lithium 1-Iododecane Undecane (90%) dimethylcopper Cangage Leaming All Righes Resorved
• Organometallic coupling reactions form carbon-carbon bonds • Used to prepare larger molecules from smaller ones Organometallic Coupling Reactions