
12-3 Preparing Alkyl Halides from Alcohols Many common methods have been developed to transform alcohols into alkyl halides Treat the alcohol with HCI,HBr,or HI Simplest method The reaction works best with tertiary alcohols,RaCOH Primary and secondary alcohols react slowly and at higher reaction temperatures -OH H-X, c H20 HH R H OH R OH OH R OH Methyl Primary Secondary Tertiary Reactivity
Many common methods have been developed to transform alcohols into alkyl halides ▪ Treat the alcohol with HCl, HBr, or HI ▪ Simplest method ▪ The reaction works best with tertiary alcohols, R3COH ▪ Primary and secondary alcohols react slowly and at higher reaction temperatures 12-3Preparing Alkyl Halides from Alcohols
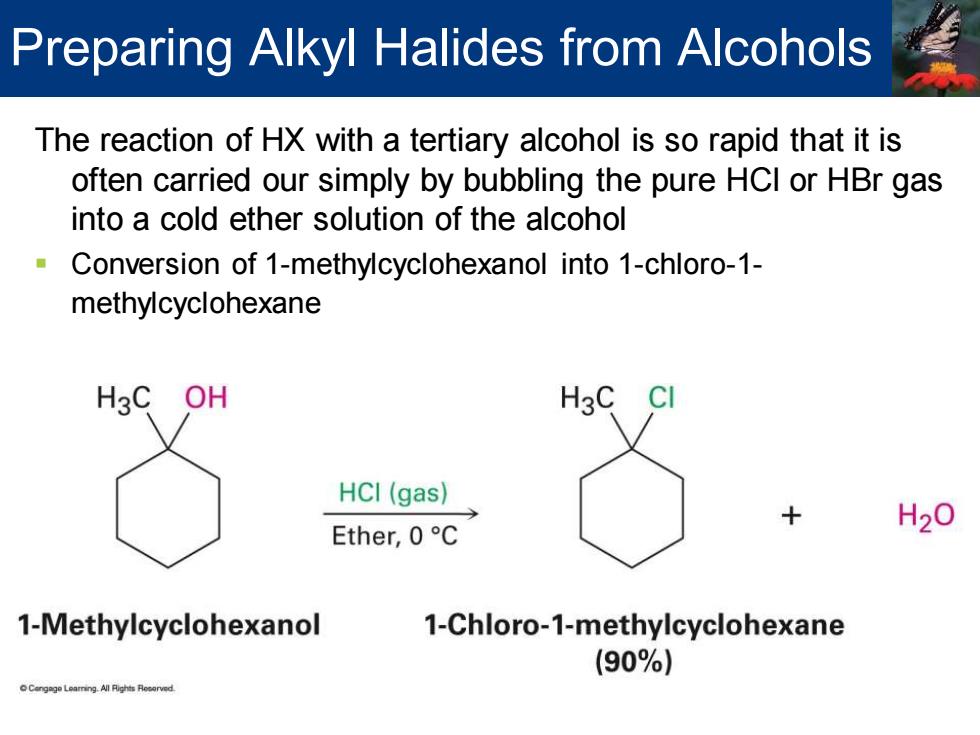
Preparing Alkyl Halides from Alcohols The reaction of HX with a tertiary alcohol is so rapid that it is often carried our simply by bubbling the pure HCI or HBr gas into a cold ether solution of the alcohol Conversion of 1-methylcyclohexanol into 1-chloro-1- methylcyclohexane HgC OH H3C HCI(gas) Ether,0C H20 1-Methylcyclohexanol 1-Chloro-1-methylcyclohexane (90%)
The reaction of HX with a tertiary alcohol is so rapid that it is often carried our simply by bubbling the pure HCl or HBr gas into a cold ether solution of the alcohol ▪ Conversion of 1-methylcyclohexanol into 1-chloro-1- methylcyclohexane Preparing Alkyl Halides from Alcohols

Preparing Alkyl Halides from Alcohols Primary and secondary alcohols are best converted into alkyl halides by treatment with either thionyl chloride (SOCl2)or phosphorus tribromide (PBr3) Reactions normally take place readily under mild conditions Reactions are less acidic and less likely to cause acid-catalyzed rearrangements than the HX method OH SOCl2 SO2+HCI Pyridine Benzoin (86%) OH Br 3 CH3CH2CHCH3 PBr3 Ether,,35℃ 3 CH3CH2CHCH3 H3PO3 Butan-2-ol 2-Bromobutane (86%)
Primary and secondary alcohols are best converted into alkyl halides by treatment with either thionyl chloride (SOCl2 ) or phosphorus tribromide (PBr3 ) ▪ Reactions normally take place readily under mild conditions ▪ Reactions are less acidic and less likely to cause acid-catalyzed rearrangements than the HX method Preparing Alkyl Halides from Alcohols

Preparing Alkyl Halides from Alcohols Alkyl fluorides can also be prepared from alcohols 0 Numerous reagents are used including diethylaminosulfur trifluoride [(CHCH2)2NSF3]and HF in pyridine solvent OH HF Pyridine Cyclohexanol Fluorocyclohexane (99%)
Alkyl fluorides can also be prepared from alcohols • Numerous reagents are used including diethylaminosulfur trifluoride [(CH3CH2 )2NSF3 ] and HF in pyridine solvent Preparing Alkyl Halides from Alcohols

12-4 Reactions of Alkyl Halides: Grignard Reagents Grignard Reagents: Named after discoverer,Victor Grignard Alkylmagnesium halide,RMgX,produced from reaction of alkyl halides,RX,with magnesium metal in ether or tetrahydrofuran (THF)solvent Examples of organometallic compounds because they contain a carbon-metal bond They can also be made from alkenyl (vinylic)and aryl (aromatic)halide 1 alkyl 2°alkyl 3°alkyl R一X Br alkenyl Halogens aryl Mg Ether or THF R-Mg-X
Grignard Reagents: ▪ Named after discoverer, Victor Grignard ▪ Alkylmagnesium halide, RMgX, produced from reaction of alkyl halides, RX, with magnesium metal in ether or tetrahydrofuran (THF) solvent ▪ Examples of organometallic compounds because they contain a carbon-metal bond ▪ They can also be made from alkenyl (vinylic) and aryl (aromatic) halide Halogens 12-4 Reactions of Alkyl Halides: Grignard Reagents