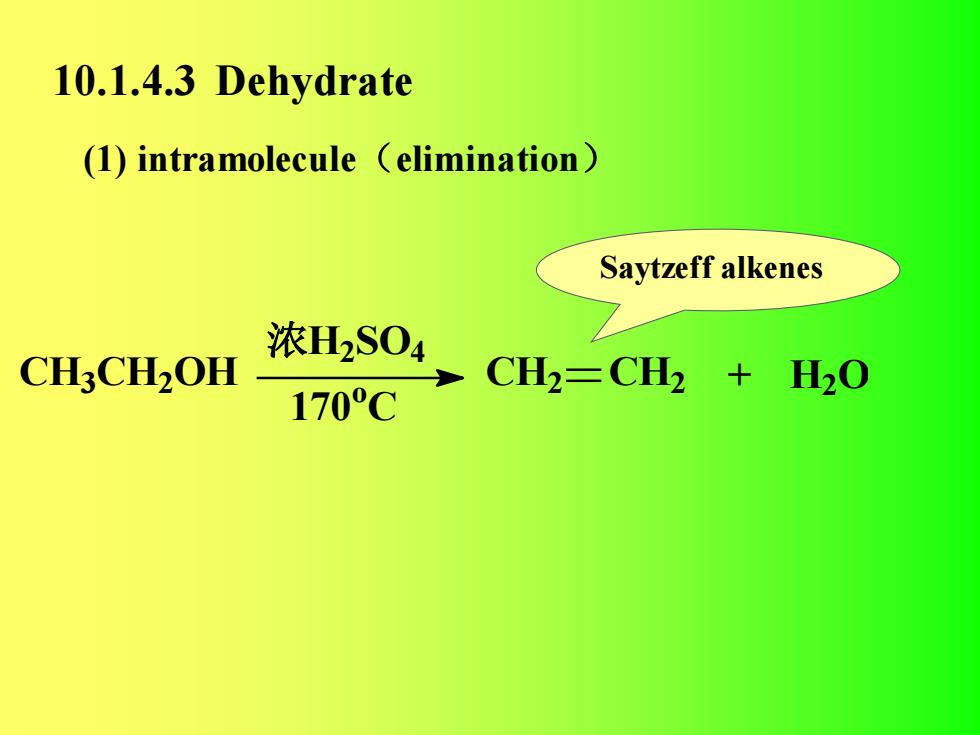
10.1.4.3 Dehydrate(1) intramolecule (elimination)Saytzeff alkenes浓H2SO4CH3CH20HCH2=CH2 2 + H0170℃
10.1.4.3 Dehydrate Saytzeff alkenes (1) intramolecule(elimination) CH3 CH2 OH 浓H2 SO4 170 o C CH2 CH2 + H2 O
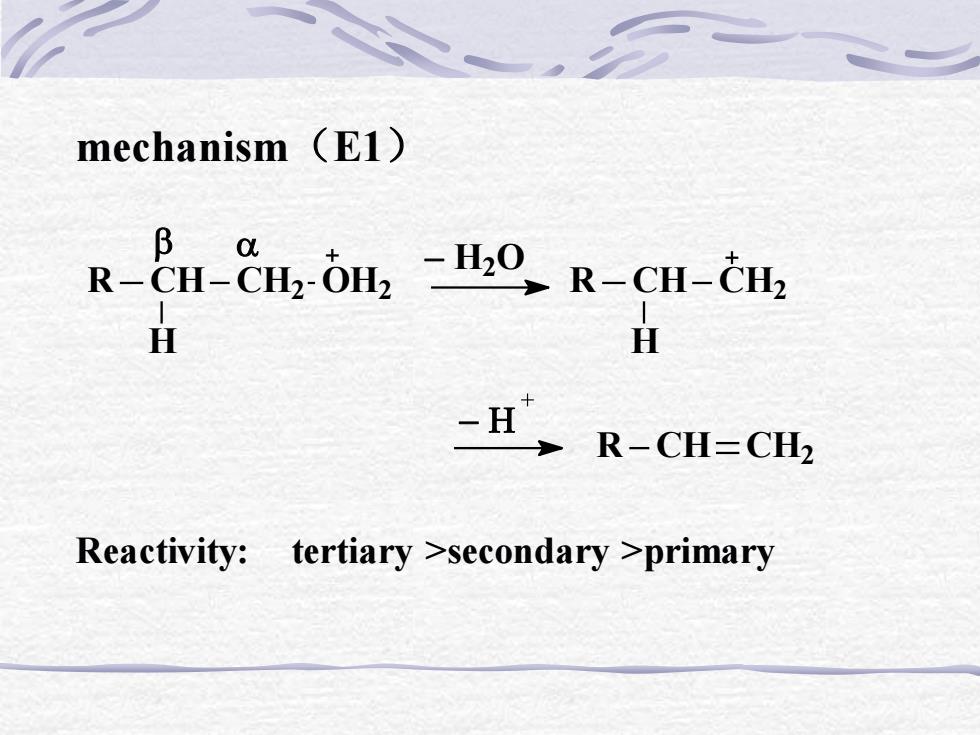
mechanism (E1)B a-H20_R-CH-cH2R-CH-CH2-OH, HH-H R-CH=CH2Reactivity: tertiary >secondary >primary
Reactivity: tertiary >secondary >primary mechanism(E1) R CH CH2 OH2 H R CH H CH2 − H2 O R CH CH2 −
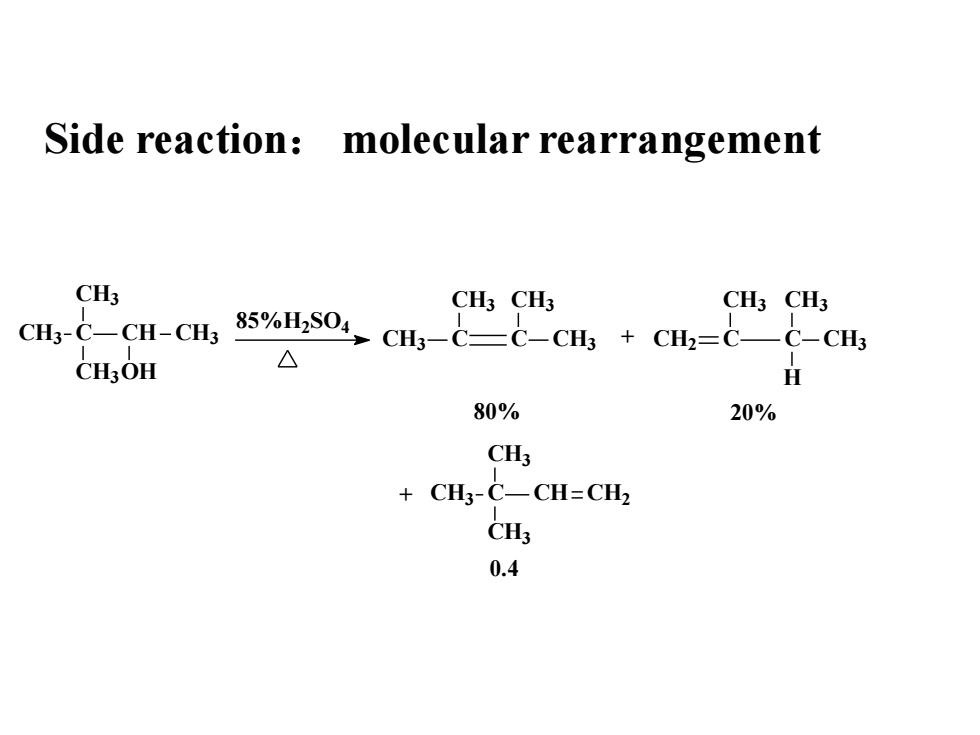
Side reaction: molecular rearrangementCH3CH3 CH3CH3 CH385%HSO4CH3-C—CH-CH3CH3-CC-CH + CH2=C—C-CH3△IHCH,OH80%20%CH3-CH=CH2+ CH3-CH30.4
Side reaction: molecular rearrangement CH3 C CH CH3 CH3 CH3OH 85%H2SO4 CH3 C C CH3 CH3 CH3 + CH2 C C CH3 CH3 CH3 H CH3 C CH CH2 CH3 CH3 80% 20% 0.4 +
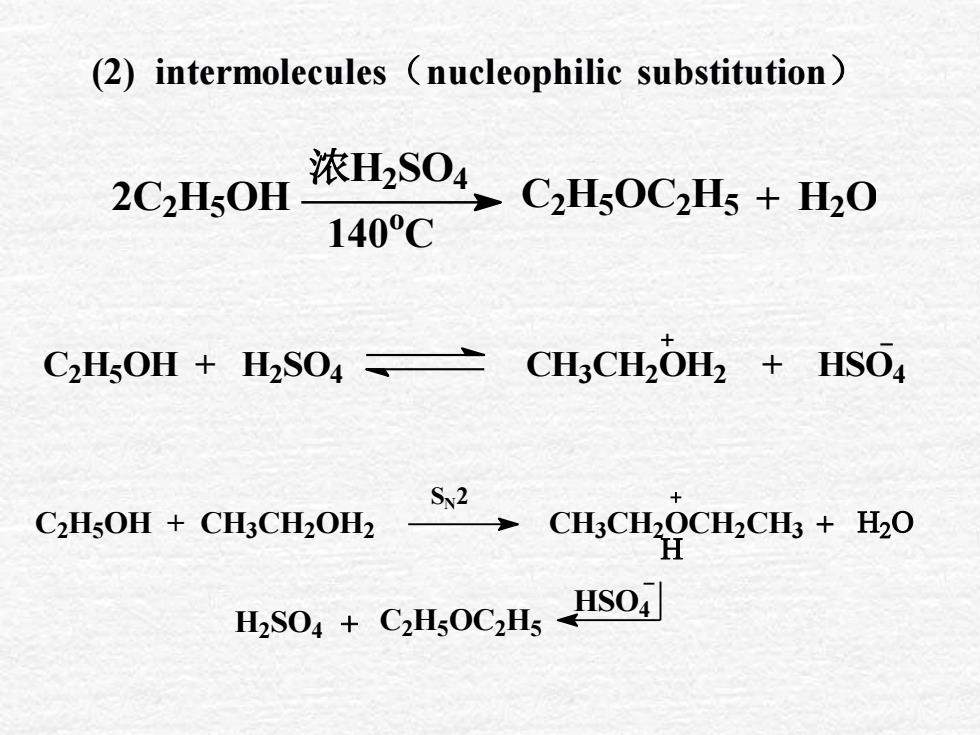
(2) intermolecules (nucleophilic substitution)浓H,SO4C,Hs0C2Hs + H202C2H,0H140CCH3CH,OH2 + HSO4C,HsOH + H2SO4 =Sv2C,HsOH + CH:CH,OH2CH,CH,OCH,CH + H20HHSO4 + CH;OCH, HSO
(2) intermolecules(nucleophilic substitution) SN2 2C2 H5 OH 浓H2 SO4 140 o C C2 H5 OC2 H5 + H2 O C2 H5 OH + H2 SO4 CH3 CH2 OH2 + HSO4 C2H5OH + CH3CH2OH2 CH3CH2OCH2CH3 + HSO4 H2 SO4 + C2H5OC2H5
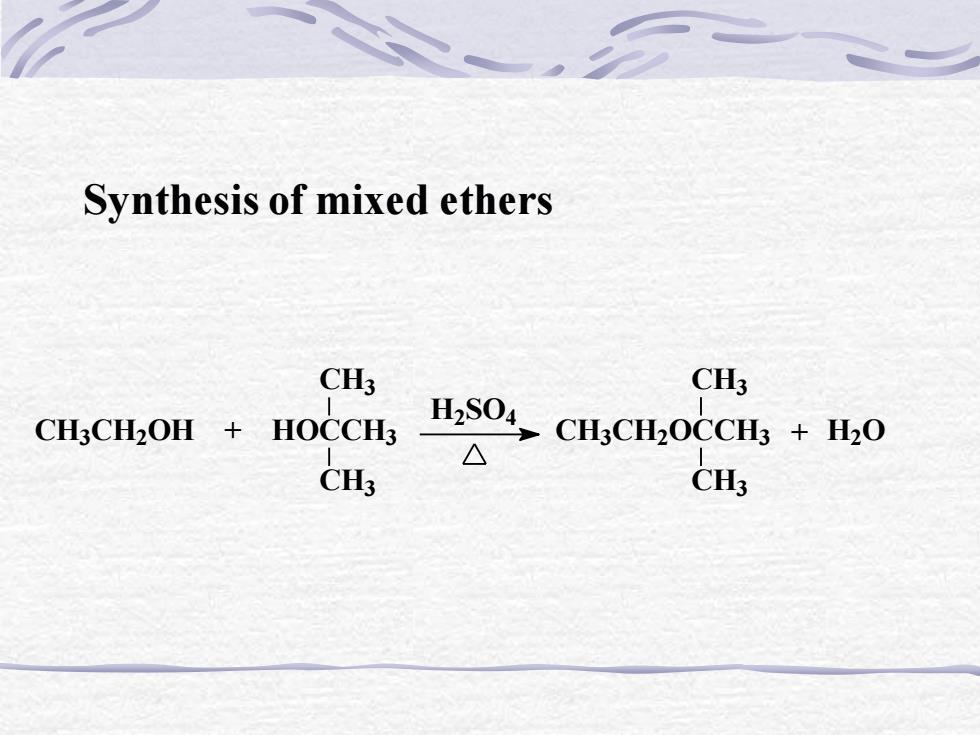
Synthesis of mixed ethersCH3CH3H,SO4CH3CH2OH + HOCCH3CH3CH,OCCH3 + H,0△CH3CH3
Synthesis of mixed ethers CH3CH2OH + HOCCH3 CH3 CH3 H2 SO4 CH3CH2OCCH3 CH3 CH3 + H2O