
Reaction Mechanism Free radical chain reaction oInitiation,propagation,termination. Br2hY→2Br. Br. HBr Br、 Br H Br + Br
Reaction Mechanism Free radical chain reaction ⚫Initiation, propagation, termination. H H Br H + HBr Br Br H Br + Br Br2 2Br h
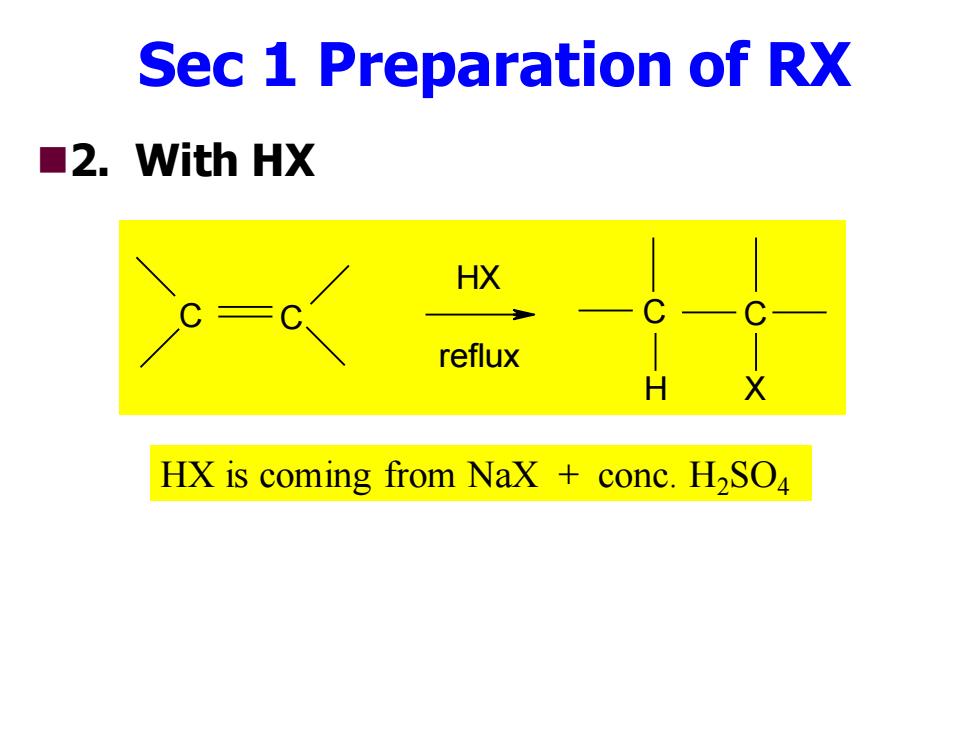
Sec 1 Preparation of RX ■2.Vith HX HX reflux HX is coming from Nax conc.H2SO
Sec 1 Preparation of RX ◼2. With HX C C HX reflux C C H X HX is coming from NaX + conc. H2SO4
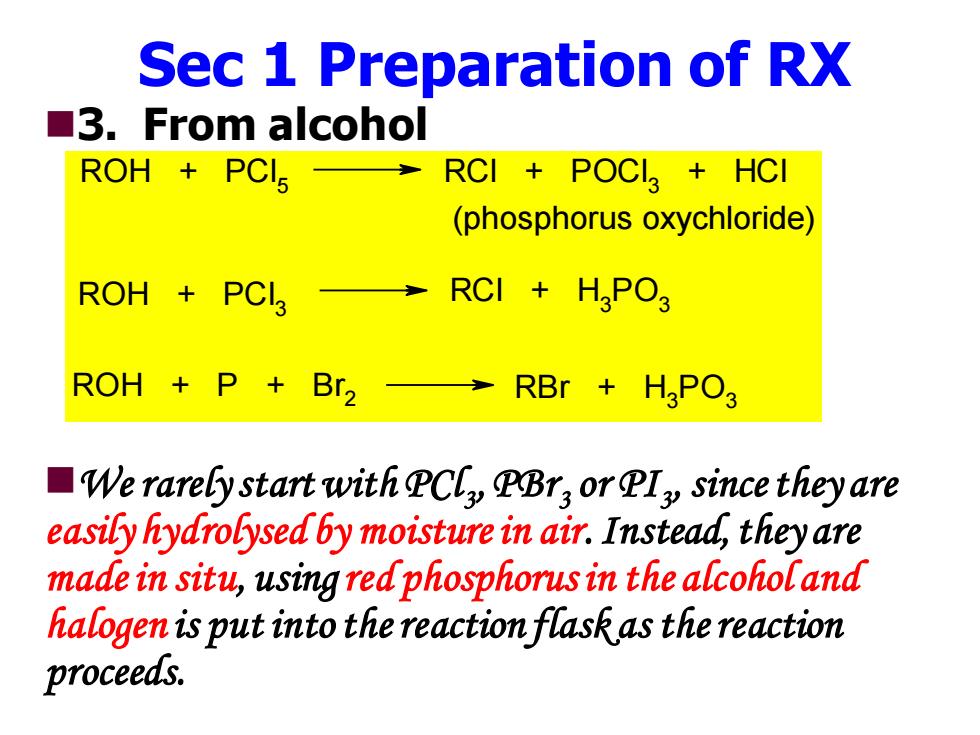
Sec 1 Preparation of RX ■3.From alcohol ROH PCls RCI POCI HCI (phosphorus oxychloride) ROH+PCL3→RCI+HPO ROH P +Br2 →RBr+H3PO3 We rarely start with PCly PBry or PIy since they are easily hydrolysed by moisture in air.Instead,they are made in situ,using red phosphorus in the alcohol and halogen is put into the reaction flask as the reaction proceeds
Sec 1 Preparation of RX ◼3. From alcohol ◼We rarely start with PCl3 , PBr3 or PI3 , since they are easily hydrolysed by moisture in air. Instead, they are made in situ, using red phosphorus in the alcohol and halogen is put into the reaction flask as the reaction proceeds. ROH + PCl 5 RCl + POCl 3 + HCl (phosphorus oxychloride) ROH + PCl 3 RCl + H3 PO3 ROH + P + Br 2 RBr + H3 PO3

Sec 1 Preparation of RX 3.From alcohol-cont. ROH+HX→RX+HO (usually HBr,HI BUT RCI cannot be prepared by this method) Pyridine (as solvent) ROH SOCI 〉 RCI SO,HCI (This product is most easily purified)
Sec 1 Preparation of RX ◼3. From alcohol – cont. ROH + HX → RX + H2O (usually HBr, HI BUT RCl cannot be prepared by this method) ROH + SOCl2 → RCl + SO2 + HCl Pyridine (as solvent) (This product is most easily purified)
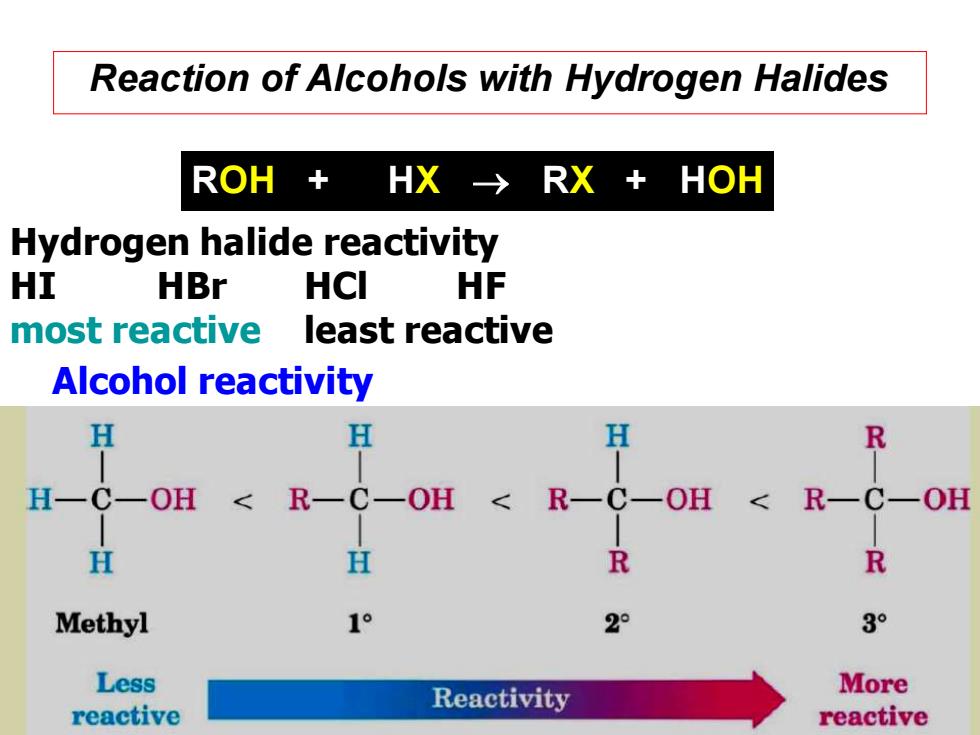
Reaction of Alcohols with Hydrogen Halides ROH+ HX-→RX+HOH Hydrogen halide reactivity HI HBr HCI HF most reactive least reactive Alcohol reactivity H H H R H一C一OH< R一C一OH < R- H H R R Methyl 1° 2° 3° Less Reactivity More reactive reactive
Reaction of Alcohols with Hydrogen Halides ROH + HX → RX + HOH Hydrogen halide reactivity HI HBr HCl HF most reactive least reactive Alcohol reactivity