
Properties of Alcohols,Phenols,and Thiols Alcohols are weak acids Do not react with weak bases such as amines or bicarbonate ion React only to a limited extent with metal hydroxides such as NaOH React with alkali metals and with strong bases such as sodium hydride (NaH)and sodium amide(NaNH2) Alkoxides are bases that are used as reagents in organic chemistry
Alcohols are weak acids ▪ Do not react with weak bases such as amines or bicarbonate ion ▪ React only to a limited extent with metal hydroxides such as NaOH ▪ React with alkali metals and with strong bases such as sodium hydride (NaH) and sodium amide (NaNH2 ) ▪ Alkoxides are bases that are used as reagents in organic chemistry Properties of Alcohols, Phenols, and Thiols
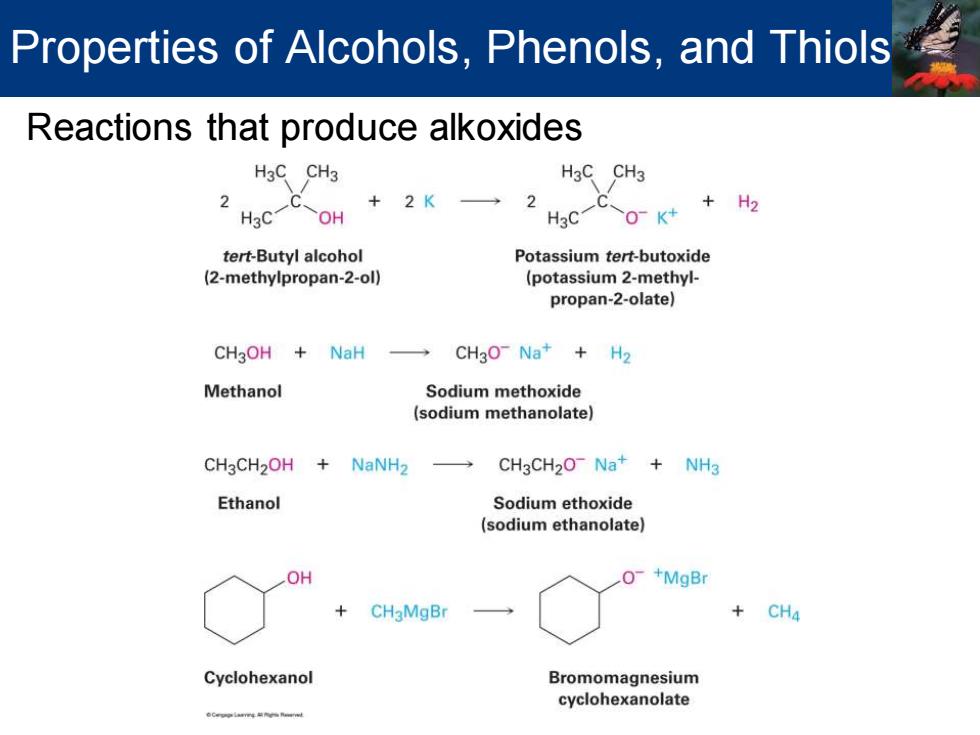
Properties of Alcohols,Phenols,and Thiols Reactions that produce alkoxides H3C CH3 H3C CH3 2 C、 +2K 2 C +H2 H3C OH H3C O-K+ tert-Butyl alcohol Potassium tert-butoxide (2-methylpropan-2-ol) (potassium 2-methyl- propan-2-olate) CH3OH NaH → CH3O-Na+H2 Methanol Sodium methoxide (sodium methanolate) CH3CH2OH NaNH2 → CH3CH20-Na+NH3 Ethanol Sodium ethoxide (sodium ethanolate) OH +MgBr CH3MgBr + CH4 Cyclohexanol Bromomagnesium cyclohexanolate oC学as件gR
Reactions that produce alkoxides Properties of Alcohols, Phenols, and Thiols
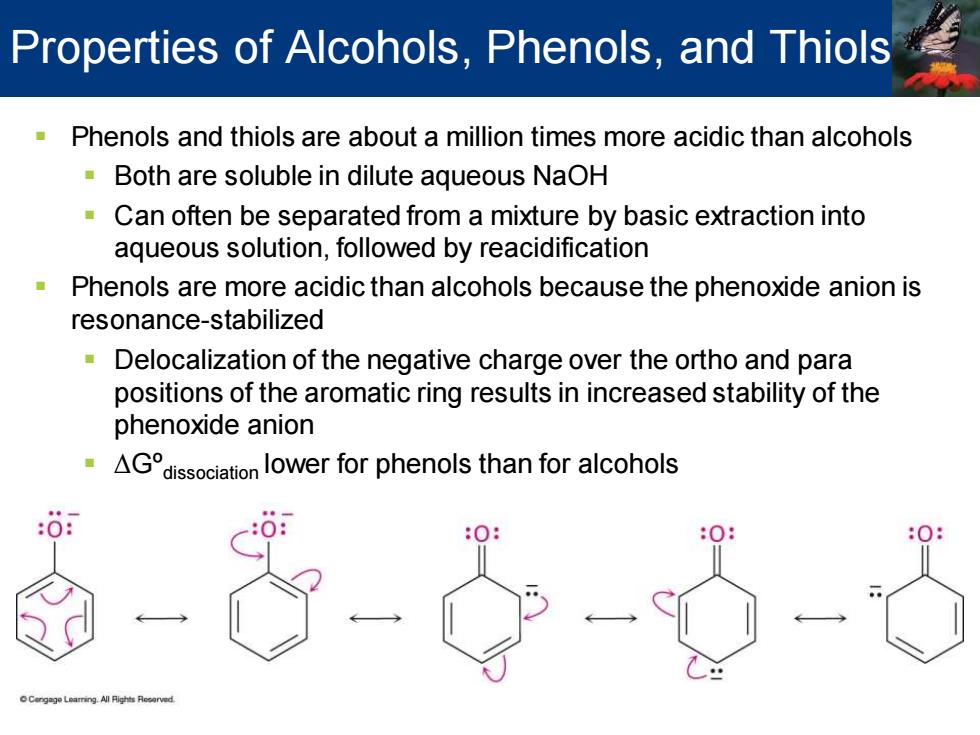
Properties of Alcohols,Phenols,and Thiols Phenols and thiols are about a million times more acidic than alcohols Both are soluble in dilute aqueous NaOH Can often be separated from a mixture by basic extraction into aqueous solution,followed by reacidification Phenols are more acidic than alcohols because the phenoxide anion is resonance-stabilized Delocalization of the negative charge over the ortho and para positions of the aromatic ring results in increased stability of the phenoxide anion AGissocation lower for phenols than for alcohols
▪ Phenols and thiols are about a million times more acidic than alcohols ▪ Both are soluble in dilute aqueous NaOH ▪ Can often be separated from a mixture by basic extraction into aqueous solution, followed by reacidification ▪ Phenols are more acidic than alcohols because the phenoxide anion is resonance-stabilized ▪ Delocalization of the negative charge over the ortho and para positions of the aromatic ring results in increased stability of the phenoxide anion ▪ ∆Gºdissociation lower for phenols than for alcohols Properties of Alcohols, Phenols, and Thiols
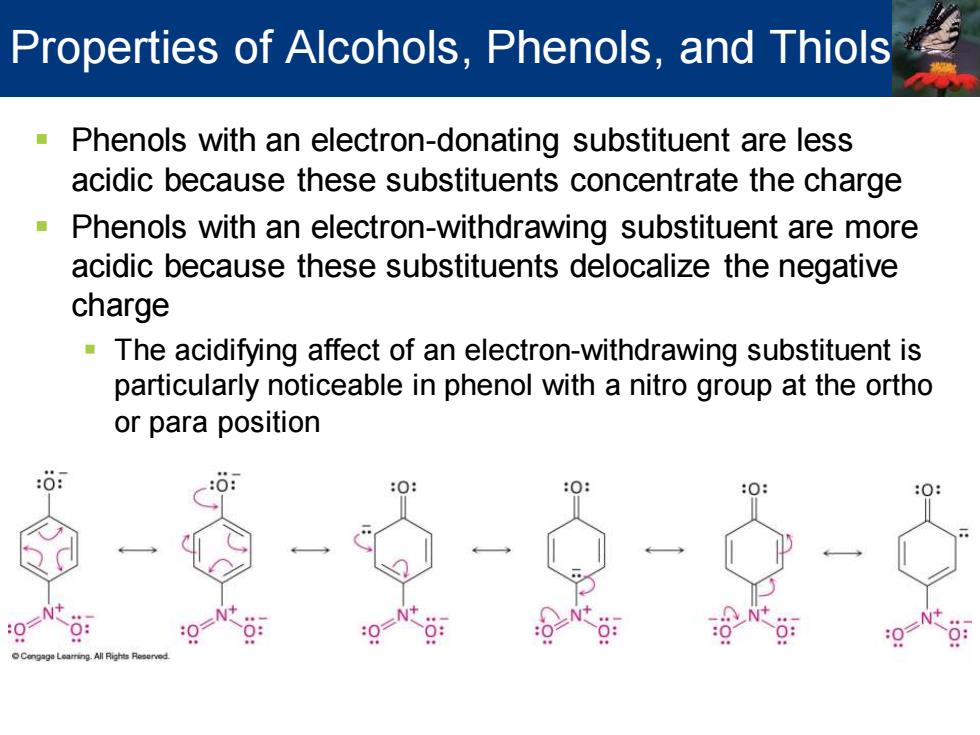
Properties of Alcohols,Phenols,and Thiols Phenols with an electron-donating substituent are less acidic because these substituents concentrate the charge Phenols with an electron-withdrawing substituent are more acidic because these substituents delocalize the negative charge The acidifying affect of an electron-withdrawing substituent is particularly noticeable in phenol with a nitro group at the ortho or para position
▪ Phenols with an electron-donating substituent are less acidic because these substituents concentrate the charge ▪ Phenols with an electron-withdrawing substituent are more acidic because these substituents delocalize the negative charge ▪ The acidifying affect of an electron-withdrawing substituent is particularly noticeable in phenol with a nitro group at the ortho or para position Properties of Alcohols, Phenols, and Thiols

Worked Example 13.1 Predicting the Relative Acidity of a Substituted Phenol Is p-hydroxybenzaldehyde more acidic or less acidic than phenol?
Is p-hydroxybenzaldehyde more acidic or less acidic than phenol? Worked Example 13.1 Predicting the Relative Acidity of a Substituted Phenol