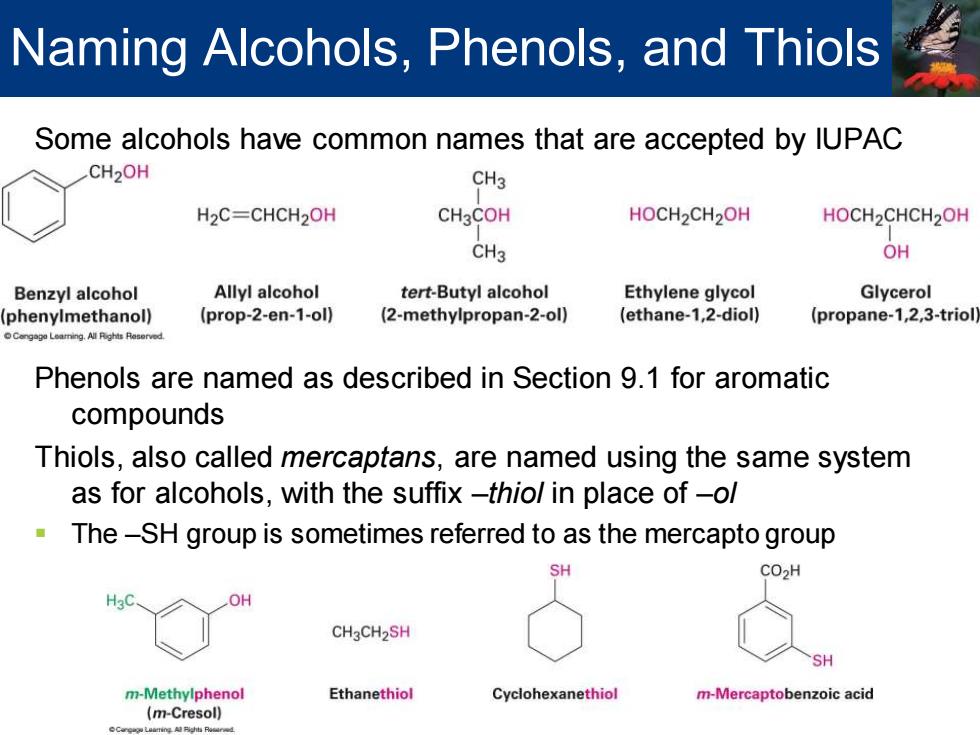
Naming Alcohols,Phenols,and Thiols Some alcohols have common names that are accepted by IUPAC CH2OH CH3 H2C=CHCH2OH CH3COH HOCH2CH2OH HOCH2CHCH2OH CH3 OH Benzyl alcohol Allyl alcohol tert-Butyl alcohol Ethylene glycol Glycerol phenylmethanol) (prop-2-en-1-ol) (2-methylpropan-2-ol) (ethane-1,2-diol) (propane-1,2,3-triol Cengago Leaming.All Pighis Reserved. Phenols are named as described in Section 9.1 for aromatic compounds Thiols,also called mercaptans,are named using the same system as for alcohols,with the suffix-thio/in place of-ol The-SH group is sometimes referred to as the mercapto group SH CO>H H OH CH3CH2SH SH m-Methylphenol Ethanethiol Cyclohexanethiol m-Mercaptobenzoic acid (m-Cresol)
Some alcohols have common names that are accepted by IUPAC Phenols are named as described in Section 9.1 for aromatic compounds Thiols, also called mercaptans, are named using the same system as for alcohols, with the suffix –thiol in place of –ol ▪ The –SH group is sometimes referred to as the mercapto group Naming Alcohols, Phenols, and Thiols
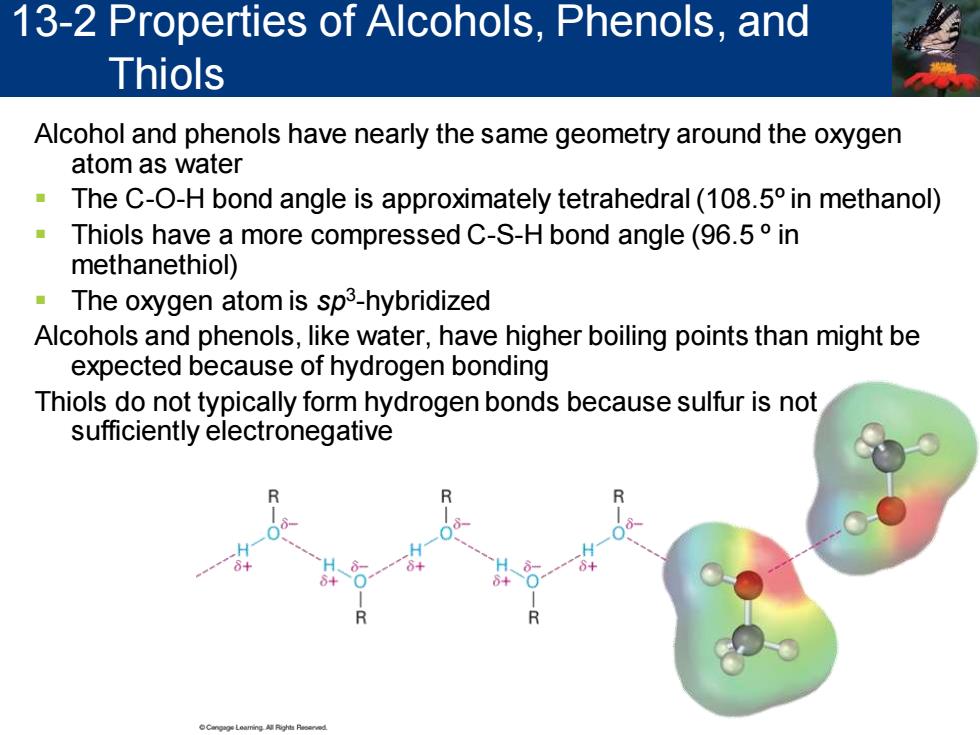
13-2 Properties of Alcohols,Phenols,and Thiols Alcohol and phenols have nearly the same geometry around the oxygen atom as water The C-O-H bond angle is approximately tetrahedral(108.5 in methanol) Thiols have a more compressed C-S-H bond angle(96.5 in methanethiol) The oxygen atom is sp3-hybridized Alcohols and phenols,like water,have higher boiling points than might be expected because of hydrogen bonding Thiols do not typically form hydrogen bonds because sulfur is not sufficiently electronegative
Alcohol and phenols have nearly the same geometry around the oxygen atom as water ▪ The C-O-H bond angle is approximately tetrahedral (108.5º in methanol) ▪ Thiols have a more compressed C-S-H bond angle (96.5 º in methanethiol) ▪ The oxygen atom is sp3 -hybridized Alcohols and phenols, like water, have higher boiling points than might be expected because of hydrogen bonding Thiols do not typically form hydrogen bonds because sulfur is not sufficiently electronegative 13-2 Properties of Alcohols, Phenols, and Thiols
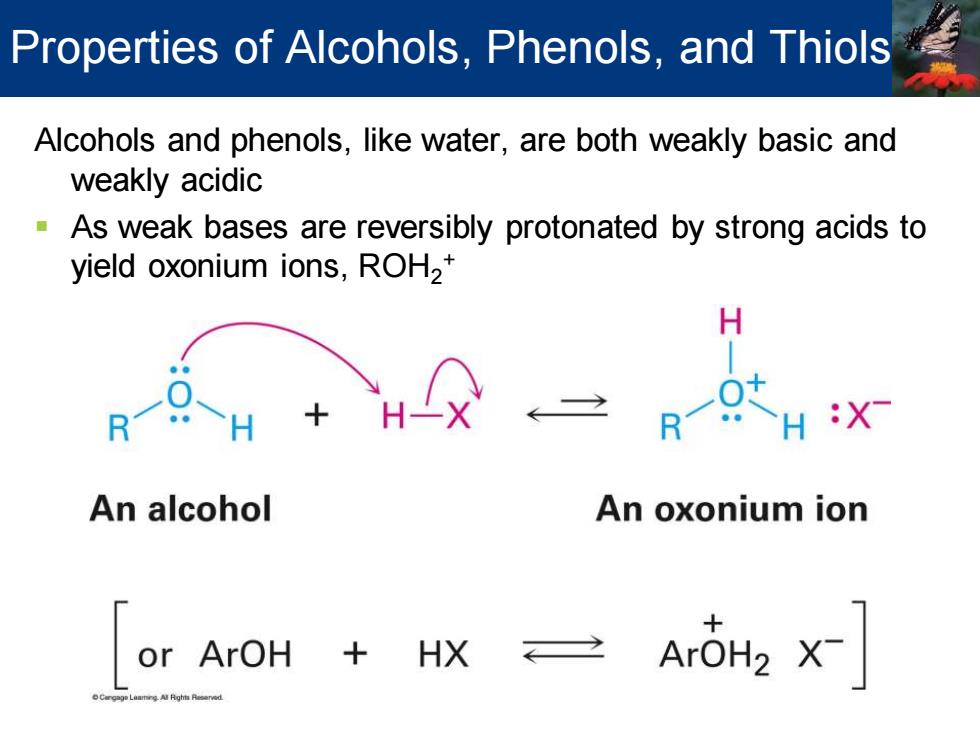
Properties of Alcohols,Phenols,and Thiols Alcohols and phenols,like water,are both weakly basic and weakly acidic As weak bases are reversibly protonated by strong acids to yield oxonium ions,ROH2* H:X An alcohol An oxonium ion or ArOH HX
Alcohols and phenols, like water, are both weakly basic and weakly acidic ▪ As weak bases are reversibly protonated by strong acids to yield oxonium ions, ROH2 + Properties of Alcohols, Phenols, and Thiols
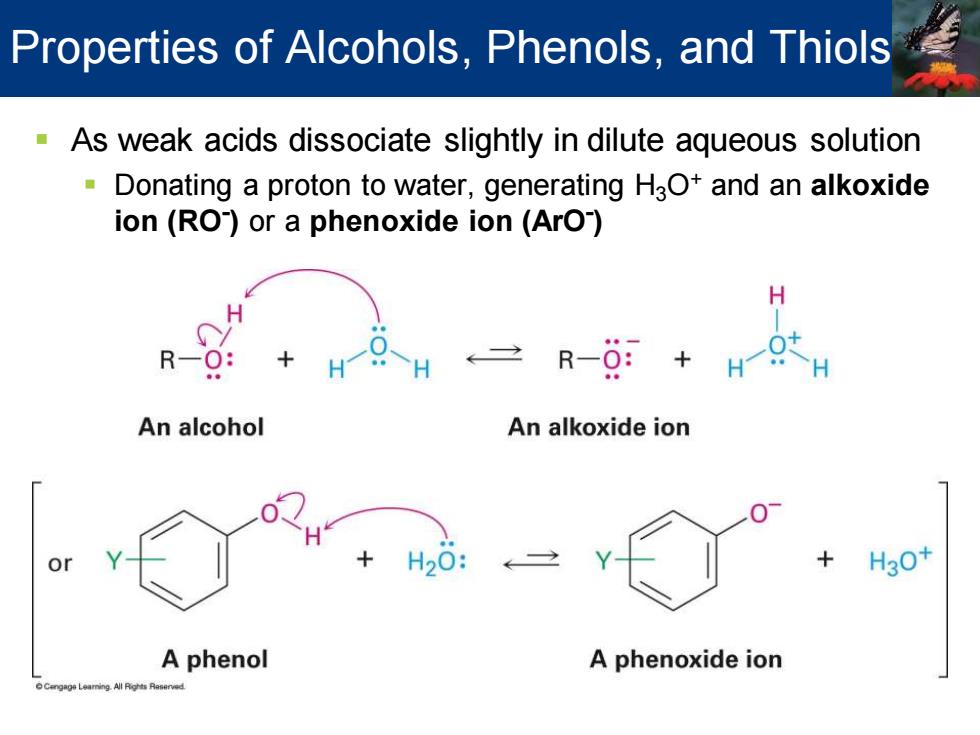
Properties of Alcohols,Phenols,and Thiols As weak acids dissociate slightly in dilute aqueous solution Donating a proton to water,generating HaO+and an alkoxide ion (RO)or a phenoxide ion(ArO) H R-0: H H R-O:+ -H An alcohol An alkoxide ion H20 H30+ A phenol A phenoxide ion
▪ As weak acids dissociate slightly in dilute aqueous solution ▪ Donating a proton to water, generating H3O+ and an alkoxide ion (RO- ) or a phenoxide ion (ArO- ) Properties of Alcohols, Phenols, and Thiols
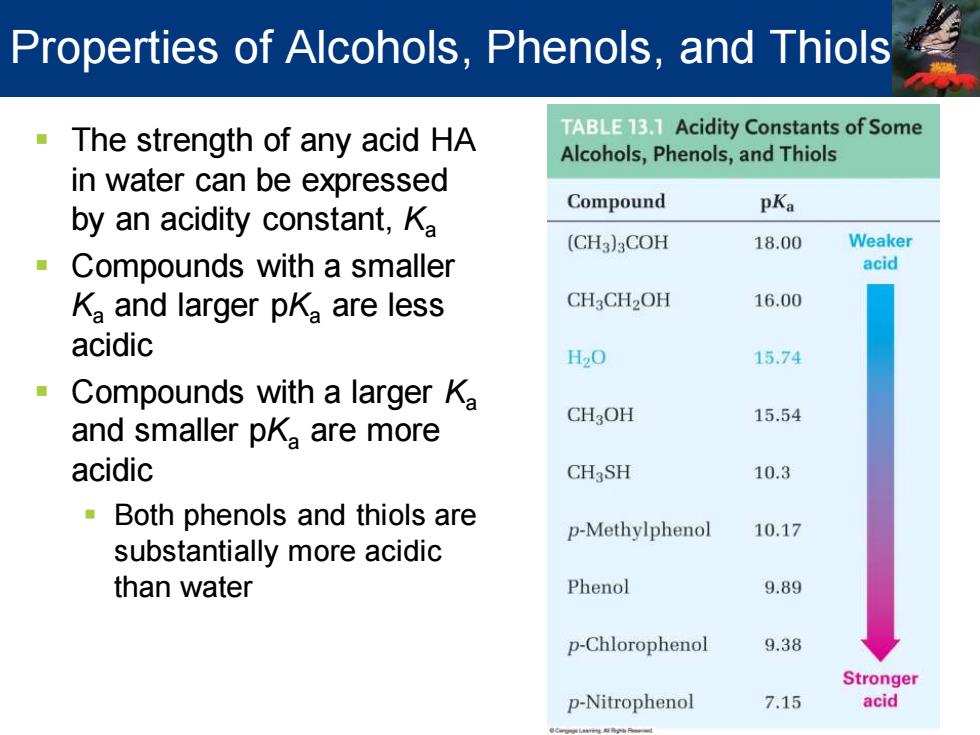
Properties of Alcohols,Phenols,and Thiols The strength of any acid HA TABLE13.1 Acidity Constants of Some Alcohols,Phenols,and Thiols in water can be expressed Compound by an acidity constant,Ka pKa (CH3)aCOH 18.00 Weaker Compounds with a smaller acid Ka and larger pKa are less CH3CH2OH 16.00 acidic H20 15.74 Compounds with a larger Ka CH3OH 15.54 and smaller pKa are more acidic CHaSH 10.3 Both phenols and thiols are p-Methylphenol 10.17 substantially more acidic than water Phenol 9.89 p-Chlorophenol 9.38 Stronger p-Nitrophenol 7.15 acid
▪ The strength of any acid HA in water can be expressed by an acidity constant, Ka ▪ Compounds with a smaller Ka and larger pKa are less acidic ▪ Compounds with a larger Ka and smaller pKa are more acidic ▪ Both phenols and thiols are substantially more acidic than water Properties of Alcohols, Phenols, and Thiols