
第1节i 醇酚醚结构特征与反应性 反应性 1、C8+-08:亲核取代, R:+I→C-O极性 (30>1) 2、o-H,B-H均有一定反应活性 -H被氧化(1°,2) B-H发生消除(C+,E1,E2) 3、一OH,-I;R-,+I效应
第1节 醇酚醚结构特征与反应性 ❖反应性 1、C+-O - : 亲核取代; R:+I C-O极性 (30>10) 2、-H, -H 均有一定反应活性 -H 被氧化(10,20) -H发生消除(C+, E1,E2) 3、-OH, -I; R-, +I效应

R一Q8 分子间氢键氢键 6 H6+ R一 6+ H Fig.3.Intermolecular hydrogen bonding between alcohols. R R R R R R R
R O H H O O R R R O H H O R R O H H O R 分子间氢键氢键
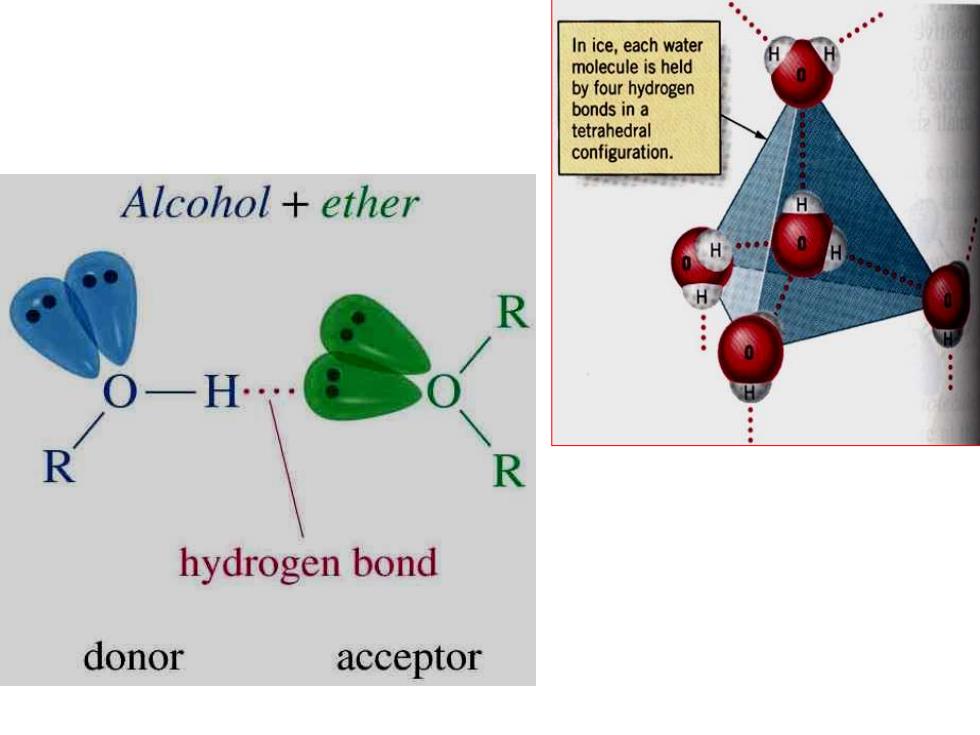
In ice,each water molecule is held by four hydrogen bonds in a tetrahedral configuration Alcohol ether R R R hydrogen bond donor acceptor
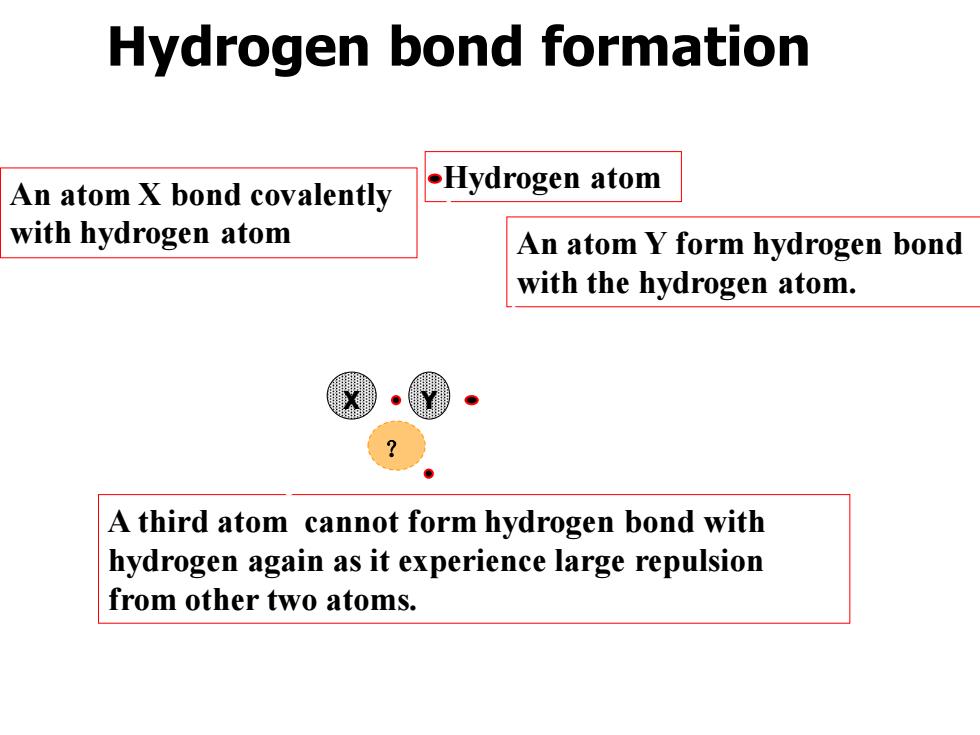
Hydrogen bond formation An atom X bond covalently .Hydrogen atom with hydrogen atom An atom Y form hydrogen bond with the hydrogen atom. A third atom cannot form hydrogen bond with hydrogen again as it experience large repulsion from other two atoms
Hydrogen bond formation X Y ? An atom X bond covalently with hydrogen atom An atom Y form hydrogen bond with the hydrogen atom. A third atom cannot form hydrogen bond with hydrogen again as it experience large repulsion from other two atoms. Hydrogen atom

8- 8 hydrogen bonding 8+ 8+ Hydrogen fluoride arrange in a zig-zag way in solid state.The hydrogen bond enthalpy is about 28 kJ/mol
Hydrogen fluoride arrange in a zig-zag way in solid state. The hydrogen bond enthalpy is about 28 kJ/mol