
dissolved in cyclohexane. Experiment2 Saturated Vapor Pressure of Pure Liquids 1.Objective 1.1 To comprehend the definition of saturated vapor pressure for pureliquids and the concept of quilibrium between gas and liquid: 1.2 To gain an insight into the relationship between temperature and the saturated vapor pressure of liquids-Clausius-Clapeyron equation. 1.3 To determine the saturated vapor pressure of cyclohexane at different temperature using vacuum gauge.to master the elementary experimental technique for low vacuum operation. 1.4 To learn the way to obtain the average molar enthalpy of vaporization of the examine liquid over a range of temperatures using graphical method as well as its normal boiling point under ambient pressure 2.Introduction The saturated vapor pressure of a pure liquid is defined as the pressure of the vapor existing in equilibrium with the liquid at a specified temperature.Here.the equilibrium state refers to a dynamic equilibrium.At a specified temperature,for a liquid system occluded in a sealed vacuum vessel the molecular species escape from the liquid surface forming the vapor phase,and meanwhile the liquid is regenerated by condensation of the vapor molecules through collision.A dynamic equilibrium will be attained if the rates of the above two processes become equal,and thereafter the density of the vapor in the gas phase will retain a fixed value.which is called the saturation vapor pressure of the liquid. The vapor pressure of a varies with temperature.Their relation cou the Clausius-Clapeyron equation
dissolved in cyclohexane. Experiment 2 Saturated Vapor Pressure of Pure Liquids 1. Objective 1.1 To comprehend the definition of saturated vapor pressure for pure liquids and the concept of equilibrium between gas and liquid; 1.2 To gain an insight into the relationship between temperature and the saturated vapor pressure of liquids—Clausius-Clapeyron equation. 1.3 To determine the saturated vapor pressure of cyclohexane at different temperature using vacuum gauge; to master the elementary experimental technique for low vacuum operation. 1.4 To learn the way to obtain the average molar enthalpy of vaporization of the examine liquid over a range of temperatures using graphical method as well as its normal boiling point under ambient pressure. 2. Introduction The saturated vapor pressure of a pure liquid is defined as the pressure of the vapor existing in equilibrium with the liquid at a specified temperature. Here, the equilibrium state refers to a dynamic equilibrium. At a specified temperature, for a liquid system occluded in a sealed vacuum vessel the molecular species escape from the liquid surface forming the vapor phase, and meanwhile the liquid is regenerated by condensation of the vapor molecules through collision. A dynamic equilibrium will be attained if the rates of the above two processes become equal, and thereafter the density of the vapor in the gas phase will retain a fixed value, which is called the saturation vapor pressure of the liquid. The vapor pressure of a pure liquid varies with temperature. Their relation could be expressed as the Clausius-Clapeyron equation

实襟 (2-2-1) Where p*is the vapor pressure of the pure liquid at temperature T.T is the thermodynamic temperature.the molar enthalpy of vaporization,R is the ideal gas constant.Within moderate range of temperature,Hcould be considered as a constant,namely,the average molar enthalpy of vaporization.Integrating the Equation(2-2-1),one can obtain: (2-2-2) RT where Cis the integration constant,which is related to the units of p* From Equation (2-2-2),it is obvious that after measuring the vapor pressures at various temperatures within a moderate temperatre angeastraight ine can be obtained by potting against 1/T.The average molar enthalpy of vaporization A.H of the liquid in this temperature range could be estimated from the slope of the straight line.The temperature at which the saturated vapor pressure of the liquid becomes equal to ambient pressure(101.325 kPa)is called the normal boiling point of the liquid Thus,the plo of the straight line can also be used toobtain the normal boiling point of the liquid. There are a number of ways to measure vapor pressures.such as the dynamic method,the static method,the saturated flow method and etc.In this experiment the static method is used,ie.,the liquid is placed in a sealed system,and then its vapor pressures at various temperatures and boiling points at various pressures are measured.This method is appropriate for measuring liquids with relatively large vapor pressures. 3.Apparatus and Reagent Apparatus for vapor pressure determination;Barometer with digital output:Thermometer(two pieces)Magnetic stirrer,Vacuum pump,Electric heater,Vacuum gauge with digital output
dT d ln p * = 2 RT V Hm (2-2−1) Where P* is the vapor pressure of the pure liquid at temperature T; T is the thermodynamic temperature; V Hm is the molar enthalpy of vaporization; R is the ideal gas constant. Within a moderate range of temperature, V Hm could be considered as a constant, namely, the average molar enthalpy of vaporization. Integrating the Equation (2-2-1), one can obtain: ln p = − R V Hm C T + 1 (2-2−2) where C is the integration constant, which is related to the units of P*. From Equation (2-2-2), it is obvious that after measuring the vapor pressures at various temperatures within a moderate temperature range, a straight line can be obtained by plotting lnP* against 1/T. The average molar enthalpy of vaporization v Hm of the liquid in this temperature range could be estimated from the slope of the straight line. The temperature at which the saturated vapor pressure of the liquid becomes equal to ambient pressure (101.325 kPa) is called the normal boiling point of the liquid. Thus, the plot of the straight line can also be used to obtain the normal boiling point of the liquid. There are a number of ways to measure vapor pressures, such as the dynamic method, the static method, the saturated flow method and etc. In this experiment the static method is used, i.e., the liquid is placed in a sealed system, and then its vapor pressures at various temperatures and boiling points at various pressures are measured. This method is appropriate for measuring liquids with relatively large vapor pressures. 3. Apparatus and Reagent Apparatus for vapor pressure determination; Barometer with digital output; Thermometer (two pieces); Magnetic stirrer; Vacuum pump; Electric heater; Vacuum gauge with digital output; Cyclohexane (AR)
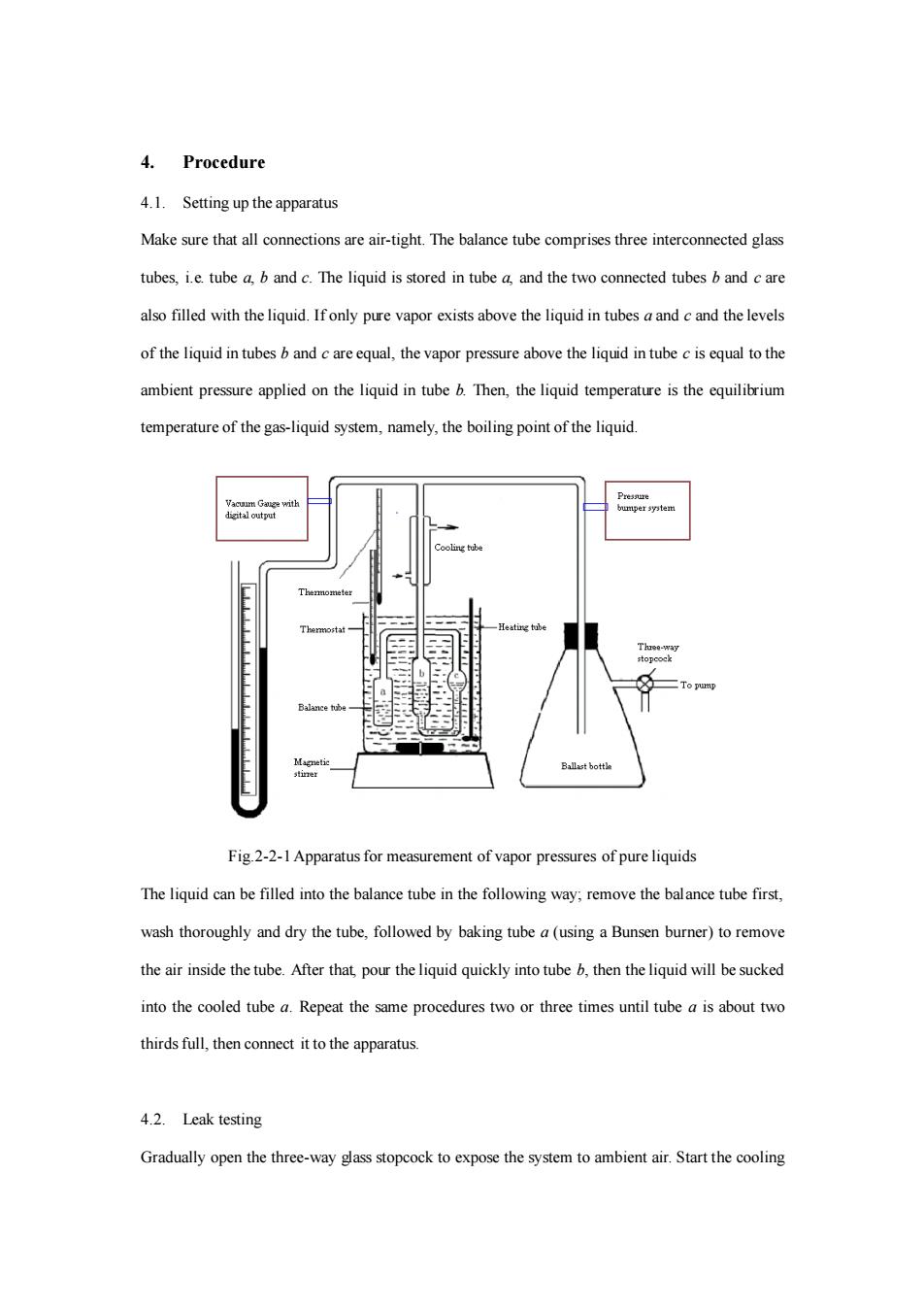
4. Procedure 4.1.Setting up the apparatus Make sure that all connections are air-tight.The balance tube comprises three interconnected glass tubes,i.e tube a.b and c.The liquid is stored in tube a and the two connected tubes b and c are afilled with theliquid.Ifony pure vapor exists above the liquid in tubesaandand the levels of the liquid in tubesand careequl,the vapor pressure above the liquid in tubecisequal to the ambient pressure applied on the liquid in tube b.Then,the liquid temperature is the equilibrium temperature of the gas-liquid system,namely,the boiling point of the liquid. Fig2-2-1 Apparatus for measurement of vapor pressures of pure liquids The liquid can be flled into the balance tube n the following way remove the balance tube first wash thoroughly and dry the tube.followed by baking tube a(using a Bunsen burner)to remove the air inside the tube.After that pour theliquid quickly into tube.then theliquid will be sucked into the cooled tube Repeat the same procedures two or three times until tube a is about two thirds full,then connect it to the apparatus. 4.2.Leak testing Gradually open the three-way glass stopcock to expose the system to ambient air.Start the cooling
4. Procedure 4.1. Setting up the apparatus Make sure that all connections are air-tight. The balance tube comprises three interconnected glass tubes, i.e. tube a, b and c. The liquid is stored in tube a, and the two connected tubes b and c are also filled with the liquid. If only pure vapor exists above the liquid in tubes a and c and the levels of the liquid in tubes b and c are equal, the vapor pressure above the liquid in tube c is equal to the ambient pressure applied on the liquid in tube b. Then, the liquid temperature is the equilibrium temperature of the gas-liquid system, namely, the boiling point of the liquid. Fig.2-2-1 Apparatus for measurement of vapor pressures of pure liquids The liquid can be filled into the balance tube in the following way; remove the balance tube first, wash thoroughly and dry the tube, followed by baking tube a (using a Bunsen burner) to remove the air inside the tube. After that, pour the liquid quickly into tube b, then the liquid will be sucked into the cooled tube a. Repeat the same procedures two or three times until tube a is about two thirds full, then connect it to the apparatus. 4.2. Leak testing Gradually open the three-way glass stopcock to expose the system to ambient air. Start the cooling

water,turn on the power,and run the vacuum pump for 4~5 min.After that,adjust the stopcock to reduce the pressure (Caution!The stopcock should be opened evenly and slowly,while paying close attention to the vacuum gauge)of the system,and close the stopcock until the system pressure attains 1x10+Pa,thus the system is vacuumized.The system can be considered leakproof if the readings on the vacuum gauge could be maintained for several minutes.If the system is leaking.a careful leak test in a section-by-section way should be carried out.Do not proceed with the experiment before assuring that the apparatus is leakproof. 4.3.Measurement of vapor pressures at various temperatures Open the system to ambient by turning the three-way stopcock.Turn on the stirrer and heat up the water bath.As the temperature increases,the liquid in the balance tube will bubble up.Keep heating the system to a temperature of about 5C above the normal boiling point of the liquid Maintain this temperature for several minutes to remove all remained air in the balance tube 4.3.1 Measuring the billing point at ambient pressure 4.3.2.Measuring vapor pressures of pure liquid at various temperatures 5.Data Analysis 5.1 Design a table for the experimental resultsand accurately report both the original data and the calculated results. 5.2 Accurate measurement of temperature is critical for this experiment The thermometer should be calibrated for the exposed stem as described in Chapter 1 of Part Three. 5.3 Polt vapor pressure pagainst temperature T. Experiment5 Phase Diagram of a binary Liquid-Vapor System 1.Objective 1.1.To draw the phase diagram of cyclohexane-ethanol binary liquid-vapor system underp and to learn the basic concept of phase diagram and the phase rule
water, turn on the power, and run the vacuum pump for 4~5 min. After that, adjust the stopcock to reduce the pressure (Caution! The stopcock should be opened evenly and slowly, while paying close attention to the vacuum gauge) of the system, and close the stopcock until the system pressure attains 1×10-4 Pa, thus the system is vacuumized. The system can be considered leakproof if the readings on the vacuum gauge could be maintained for several minutes. If the system is leaking, a careful leak test in a section-by-section way should be carried out. Do not proceed with the experiment before assuring that the apparatus is leakproof. 4.3. Measurement of vapor pressures at various temperatures Open the system to ambient by turning the three-way stopcock. Turn on the stirrer and heat up the water bath. As the temperature increases, the liquid in the balance tube will bubble up. Keep heating the system to a temperature of about 5℃ above the normal boiling point of the liquid. Maintain this temperature for several minutes to remove all remained air in the balance tube. 4.3.1 Measuring the billing point at ambient pressure 4.3.2. Measuring vapor pressures of pure liquid at various temperatures 5. Data Analysis 5.1 Design a table for the experimental results, and accurately report both the original data and the calculated results. 5.2 Accurate measurement of temperature is critical for this experiment. The thermometer should be calibrated for the exposed stem as described in Chapter 1 of Part Three. 5.3 Polt vapor pressure p* against temperature T. Experiment 5 Phase Diagram of a binary Liquid-Vapor System 1. Objective 1.1. To draw the phase diagram of cyclohexane-ethanol binary liquid-vapor system under p$, and to learn the basic concept of phase diagram and the phase rule

1.2.To learn the method of detemmining normal boiling point and the boiling point of a binary liquid system. 1.3.To learn the method of determining the composition of binary liquid system by refractometry. 2.Introduction 2.1.Phase diagram ofliquid-vapor system A two-component liquid system is composed of a mixture of two kinds of liquid If the two components are miscible in all proportions,it is called a totally miscible liquid-liquid system.The boiling point of aliquid is the temperature at which the vapor pressure of the liquid has a define value.However,the boiling point of a two-component liquid depends not only on the ambient pressure but also on the composition of the liquid. 22.Ebulliometer Different ebulliometers may have there own features,but they are all designed for obtaining the boiling point,analyzing the samples conveniently and preventing the samplefrom over-heatingor fractionation. 2.3.Composition analysis Cyclohexane and ethanol are chosen as the liquid samples in this experiment.Their refractive indexes are sufficiently different from each other,and only a very small amount of sample is needed for refractometry.Therefore phase composition an be obtained through theuse of a calibration curve of refractive index as a function of composition. 3. Apparatus and Reagent Ebulliometer,Mercury-in-glass thermometer(50-l0o℃,±O.1℃方Mercury-.in-glass thermometer(0~100C,1C);Voltage transformer(0.5kVA);Digital Abbe refractometer,Ultra thermostatic water-circulator bath;Cyclohexane(AR):Absolute ethanol(AR)Double distilled water,Ice. 4.Procedure 4.1.Making the calibration curve 4.2. Installing the ebulliometer
1.2. To learn the method of determining normal boiling point and the boiling point of a binary liquid system. 1.3. To learn the method of determining the composition of binary liquid system by refractometry. 2. Introduction 2.1. Phase diagram of liquid-vapor system A two-component liquid system is composed of a mixture of two kinds of liquid. If the two components are miscible in all proportions, it is called a totally miscible liquid-liquid system. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid has a define value. However, the boiling point of a two-component liquid depends not only on the ambient pressure but also on the composition of the liquid. 2.2. Ebulliometer Different ebulliometers may have there own features, but they are all designed for obtaining the boiling point correctly, analyzing the samples conveniently and preventing the sample from over-heating or fractionation. 2.3. Composition analysis Cyclohexane and ethanol are chosen as the liquid samples in this experiment. Their refractive indexes are sufficiently different from each other, and only a very small amount of sample is needed for refractometry. Therefore, the equilibrium phase composition can be obtained through the use of a calibration curve of refractive index as a function of composition. 3. Apparatus and Reagent Ebulliometer; Mercury-in-glass thermometer(50~100 ℃,± 0.1 ℃ ); Mercury-in-glass thermometer(0~100℃,±0.1℃); Voltage transformer(0.5kVA); Digital Abbe refractometer; Ultra thermostatic water-circulator bath; Cyclohexane(AR); Absolute ethanol(AR); Double distilled water; Ice. 4. Procedure 4.1. Making the calibration curve 4.2. Installing the ebulliometer