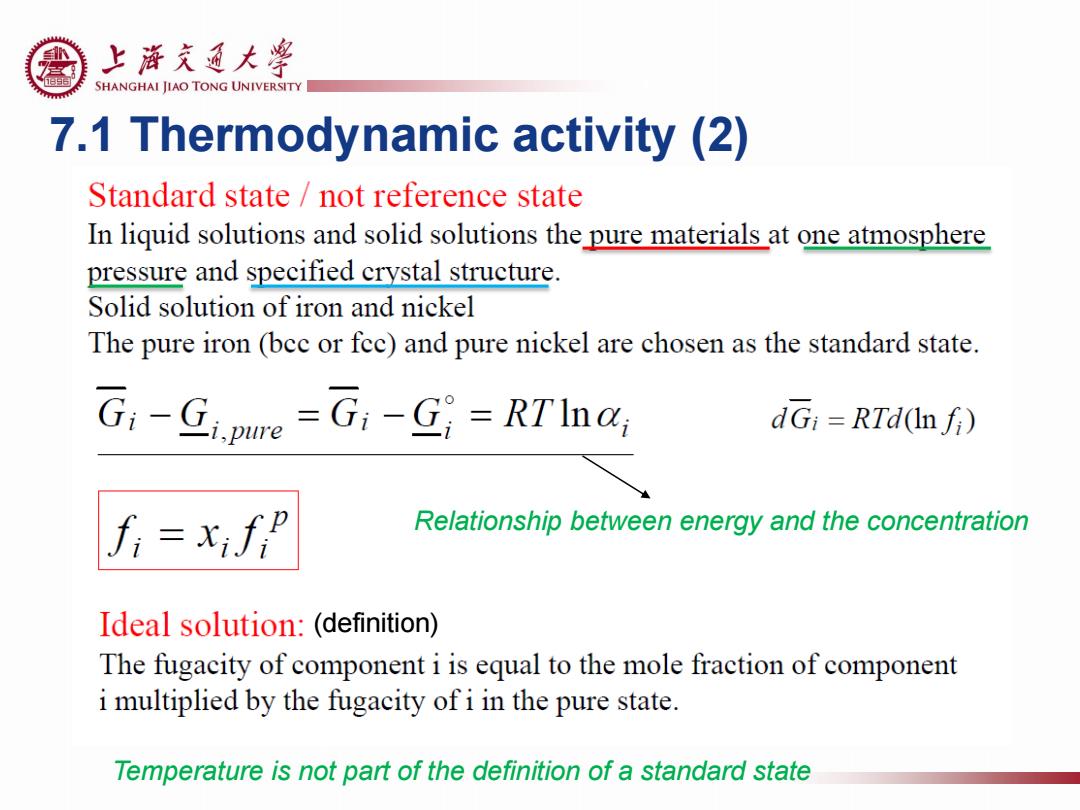
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY 7.1 Thermodynamic activity (2) Standard state not reference state In liquid solutions and solid solutions the pure materials at one atmosphere pressure and specified crystal structure. Solid solution of iron and nickel The pure iron (bec or fec)and pure nickel are chosen as the standard state. G:-Gjpure =G1-G;=RTInar dGi =RTd(Infi) Relationship between energy and the concentration Ideal solution:(definition) The fugacity of component i is equal to the mole fraction of component i multiplied by the fugacity of i in the pure state. Temperature is not part of the definition of a standard state
7.1 Thermodynamic activity (2) Temperature is not part of the definition of a standard state Relationship between energy and the concentration (definition)
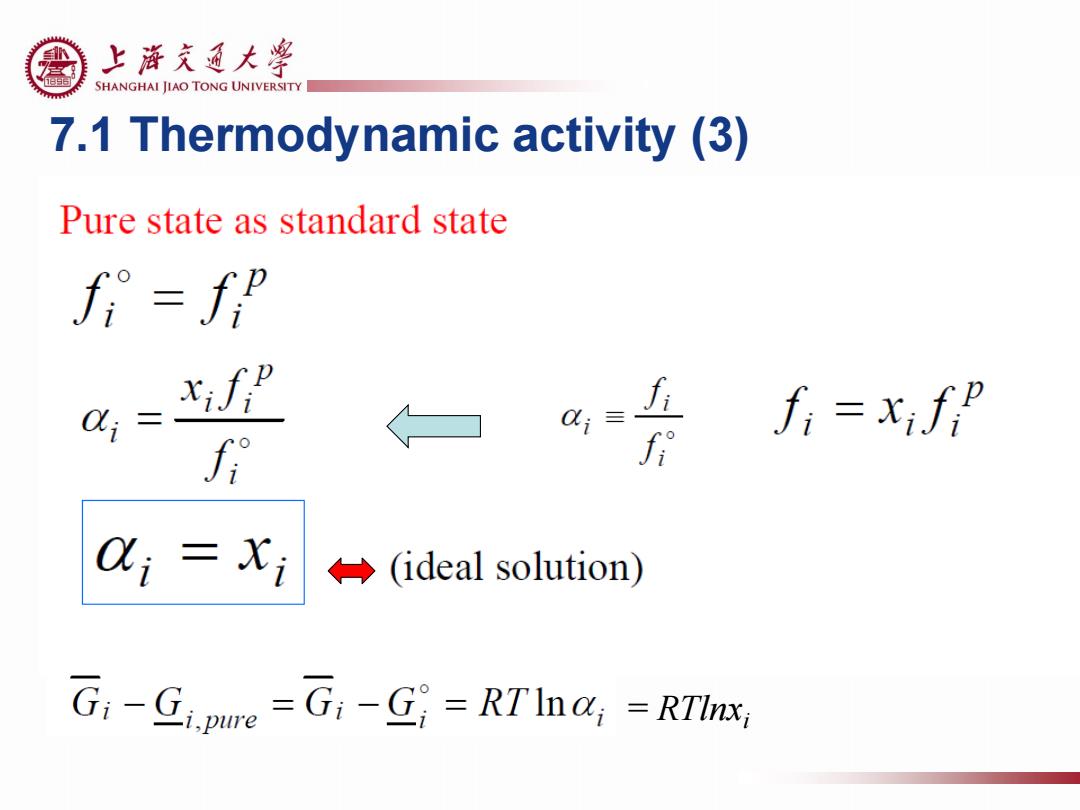
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY 7.1 Thermodynamic activity (3) Pure state as standard state fi=fp xifp fi=xif f i(ideal solution) G:-Gipue =G:-G=RTIna;-RTInx
7.1 Thermodynamic activity (3) = RTlnxi
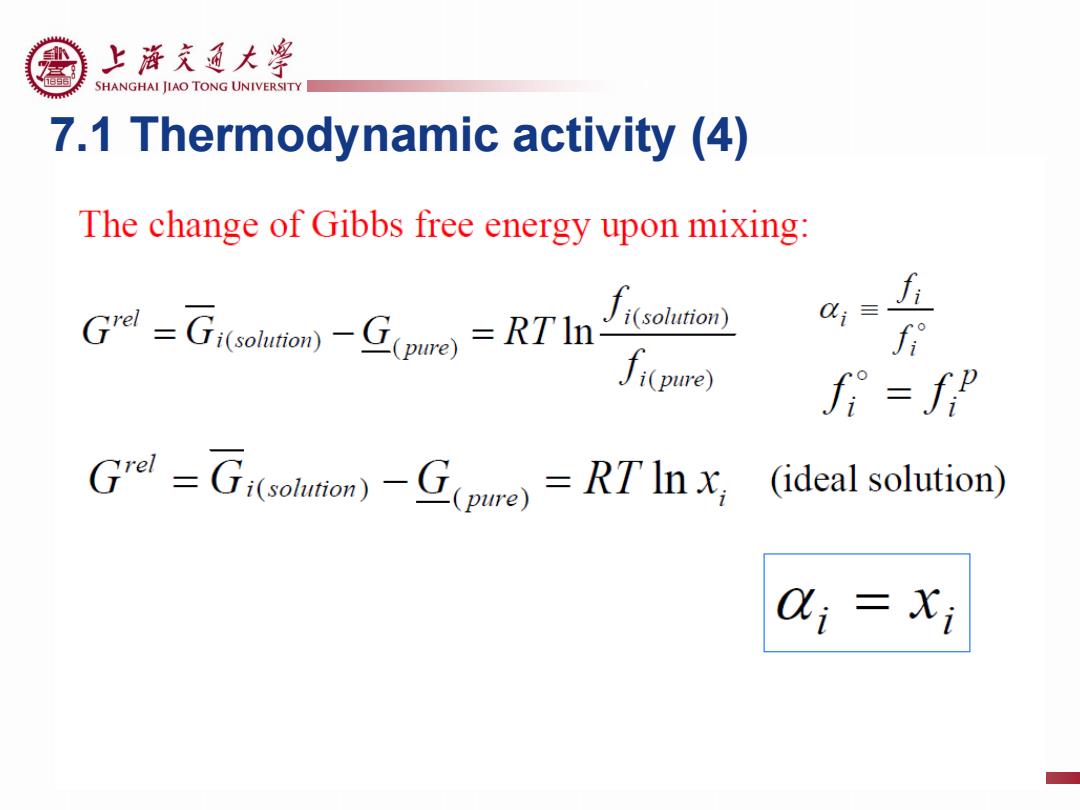
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY 7.1 Thermodynamic activity (4) The change of Gibbs free energy upon mixing: i-RTh solution)) f Ji(pure) f =f” Grd=Gi(sohmam)-G(pue)=RTInx (ideal solution) 0;=i
7.1 Thermodynamic activity (4)

上游充通大粤 e SHANGHAI JIAO TONG UNIVERSITY 7.1 Thermodynamic activity (5) f° 1.0 0 0 Pure x1> Figure 7.1 Fugacity of component i(f)and activity of component i(a,)as a function of mole fraction (x,)for an ideal solution
7.1 Thermodynamic activity (5)
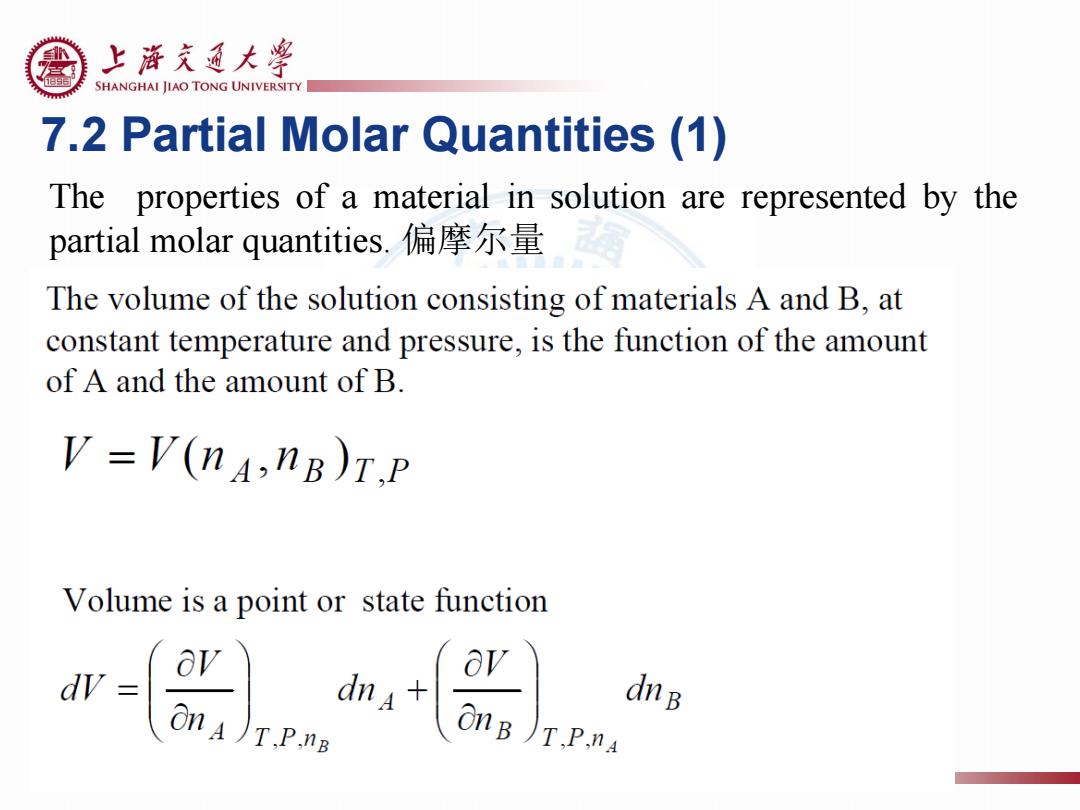
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY 7.2 Partial Molar Quantities (1) The properties of a material in solution are represented by the partial molar quantities..偏摩尔量 The volume of the solution consisting of materials A and B,at constant temperature and pressure,is the function of the amount of A and the amount of B V =V(nA,nB)T.P Volume is a point or state function dV av dnB OnA)T.P.nB
7.2 Partial Molar Quantities (1) The properties of a material in solution are represented by the partial molar quantities. 偏摩尔量