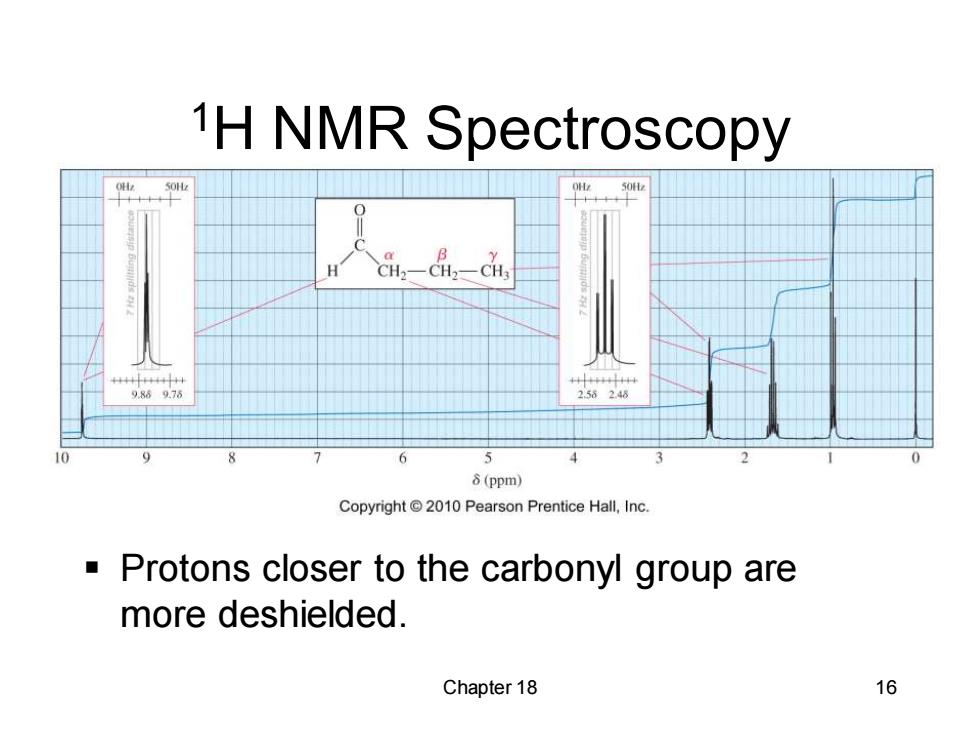
1H NMR Spectroscopy 50 9.86976 2.58248 8(ppm) Copyright2010 Pearson Prentice Hall,Inc. Protons closer to the carbonyl group are more deshielded. Chapter 18 16
Chapter 18 16 1H NMR Spectroscopy ▪ Protons closer to the carbonyl group are more deshielded
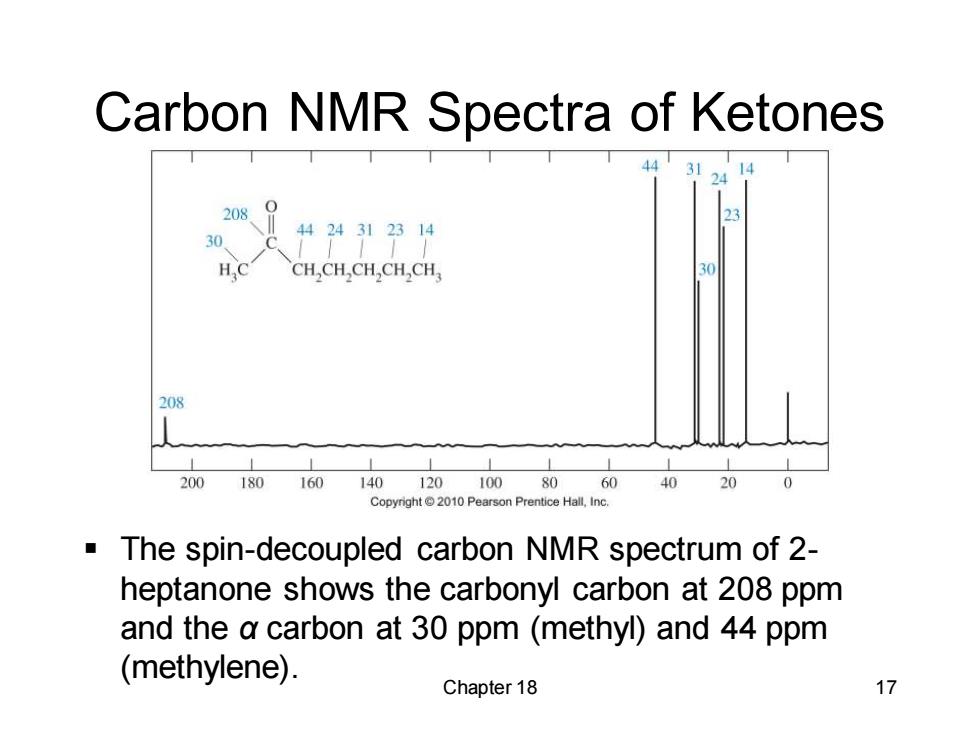
Carbon NMR Spectra of Ketones 44 31 14 208、 30 4424312314 CH,CH,CH,CH,CH 30 208 200180160 14012010080604020 0 Copyright2010 Pearson Prentice Hall,Inc. The spin-decoupled carbon NMR spectrum of 2- heptanone shows the carbonyl carbon at 208 ppm and the a carbon at 30 ppm (methyl)and 44 ppm (methylene). Chapter 18 17
Chapter 18 17 Carbon NMR Spectra of Ketones ▪ The spin-decoupled carbon NMR spectrum of 2- heptanone shows the carbonyl carbon at 208 ppm and the α carbon at 30 ppm (methyl) and 44 ppm (methylene)
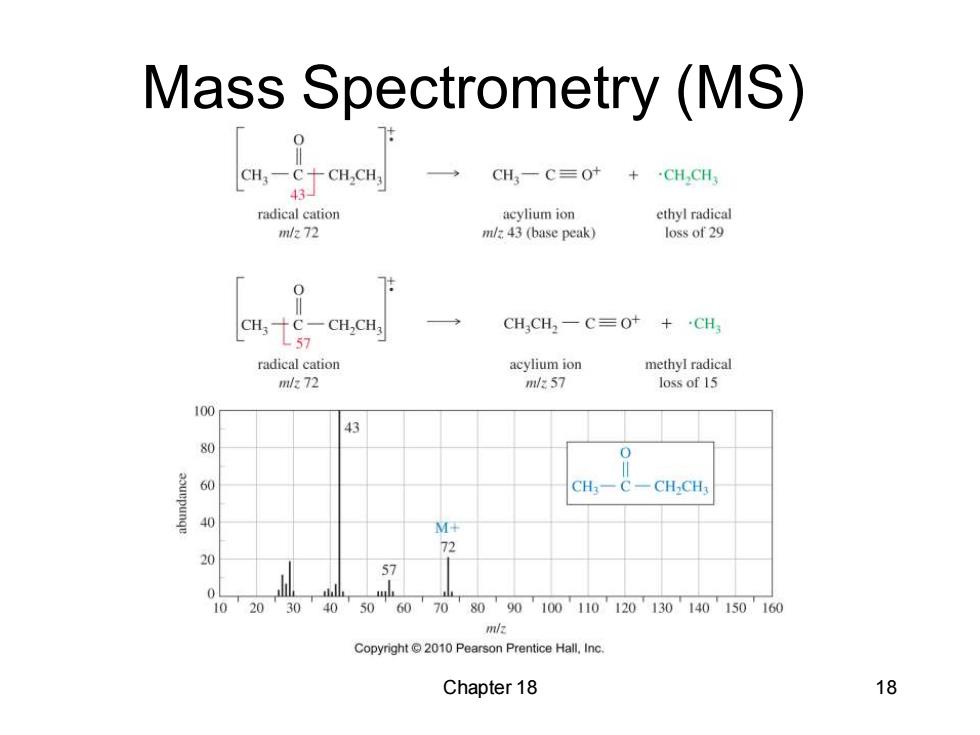
Mass Spectrometry (MS) CH,一CCH,CH, CH,一C=Ot+CH,CH 43 radical cation acylium ion ethyl radical m/272 m/z.43 (base peak) loss of 29 7: CH,十C-CH,CH, CHCH2-C=0+ +CH3 L57 radical cation acylium ion methyl radical ml/72 m/57 loss of 15 100 43 80 0 60 CH-CH3 40 M+ 72 20 57 10 20 30 40 50 60 70 8090100110120130140150160 Copyright2010 Pearson Prentice Hall,Inc. Chapter 18 18
Chapter 18 18 Mass Spectrometry (MS)
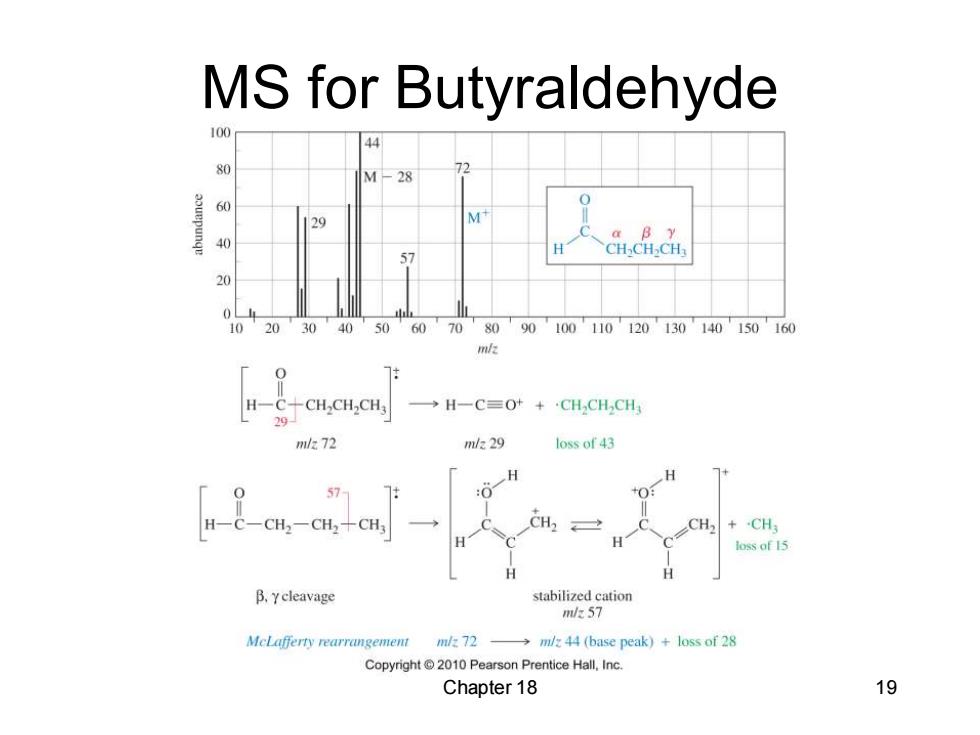
MS for Butyraldehyde 100 44 80 M-28 6( 9 a B y 40 57 CH-CH>CH 10 20 30 40 50 60 70 80 90100110120130140150160 m/z H一C+CH,CHCH →H一C=Ot+CH,CH,CH 7m/272 m/229 loss of 43 H : H一C-CH2-CH2+CH3 CH CH loss of 15 B.ycleavage stabilized cation m/k57 MeLafferty rearrangement m/z 72 -m/z 44 (base peak)+loss of 28 Copyright 2010 Pearson Prentice Hall,Inc. Chapter 18 19
Chapter 18 19 MS for Butyraldehyde
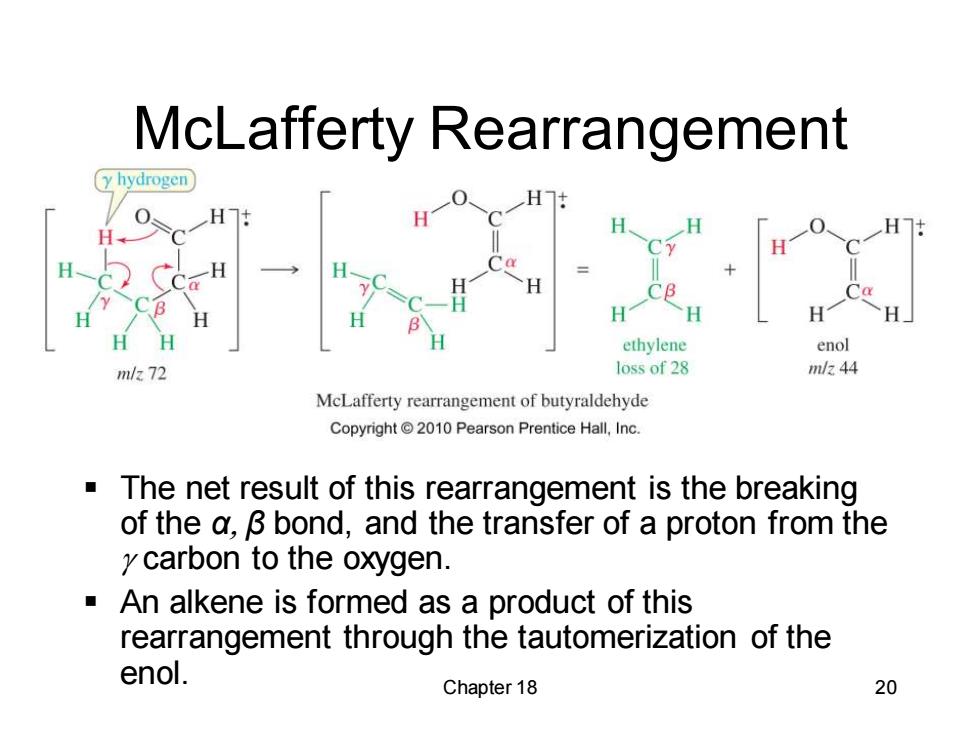
McLafferty Rearrangement (y hydrogen H H-C CE H CB H H ethylene enol m/z 72 loss of 28 mlz 44 McLafferty rearrangement of butyraldehyde Copyright 2010 Pearson Prentice Hall,Inc. The net result of this rearrangement is the breaking of the a,B bond,and the transfer of a proton from the y carbon to the oxygen. An alkene is formed as a product of this rearrangement through the tautomerization of the enol. Chapter 18 20
Chapter 18 20 McLafferty Rearrangement ▪ The net result of this rearrangement is the breaking of the α, β bond, and the transfer of a proton from the carbon to the oxygen. ▪ An alkene is formed as a product of this rearrangement through the tautomerization of the enol