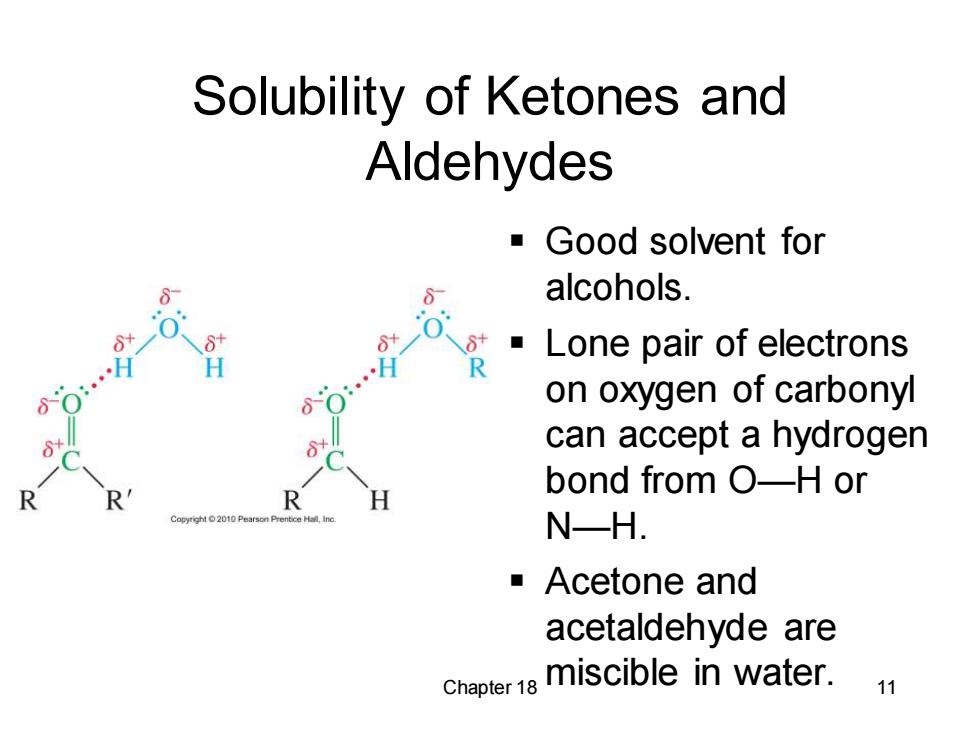
Solubility of Ketones and Aldehydes Good solvent for alcohols. 0 Lone pair of electrons H H R 80 on oxygen of carbonyl can accept a hydrogen bond from O-H or R R H N-H. -Acetone and acetaldehyde are chapter1e miscible in water. 11
Chapter 18 11 Solubility of Ketones and Aldehydes ▪ Good solvent for alcohols. ▪ Lone pair of electrons on oxygen of carbonyl can accept a hydrogen bond from O—H or N—H. ▪ Acetone and acetaldehyde are miscible in water
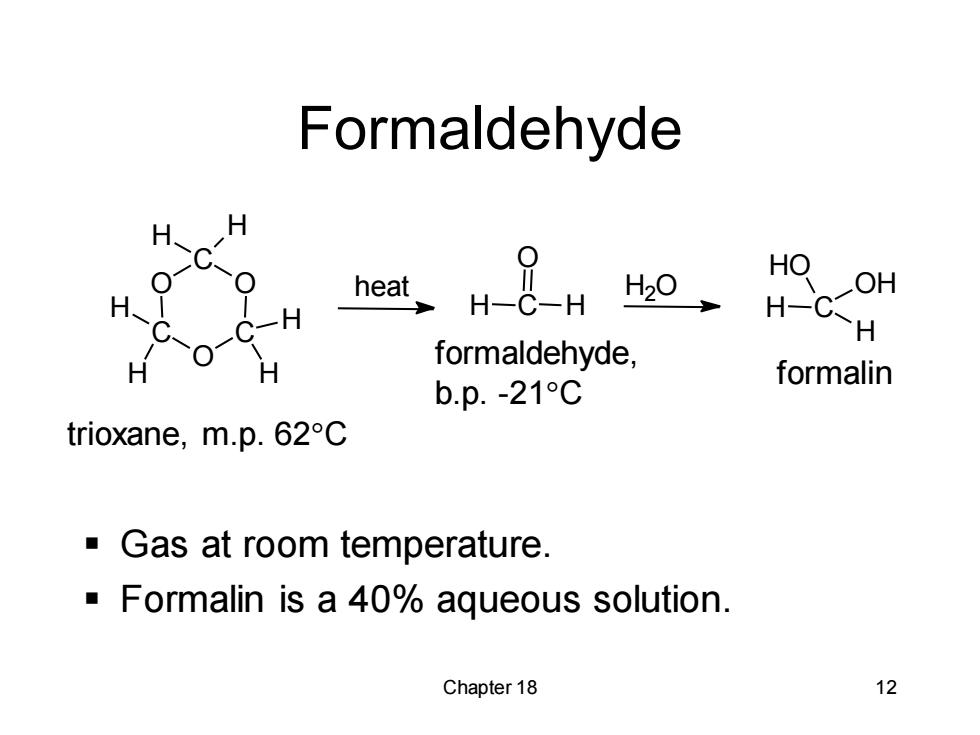
Formaldehyde HO OH H-C-H formaldehyde, b.p.-21C formalin trioxane,m.p.62°C Gas at room temperature. Formalin is a 40%aqueous solution. Chapter 18 12
Chapter 18 12 Formaldehyde ▪ Gas at room temperature. ▪ Formalin is a 40% aqueous solution. trioxane, m.p. 62C formaldehyde, b.p. -21C formalin O C O C O C H H H H H H heat H C O H H2O H C H OH HO
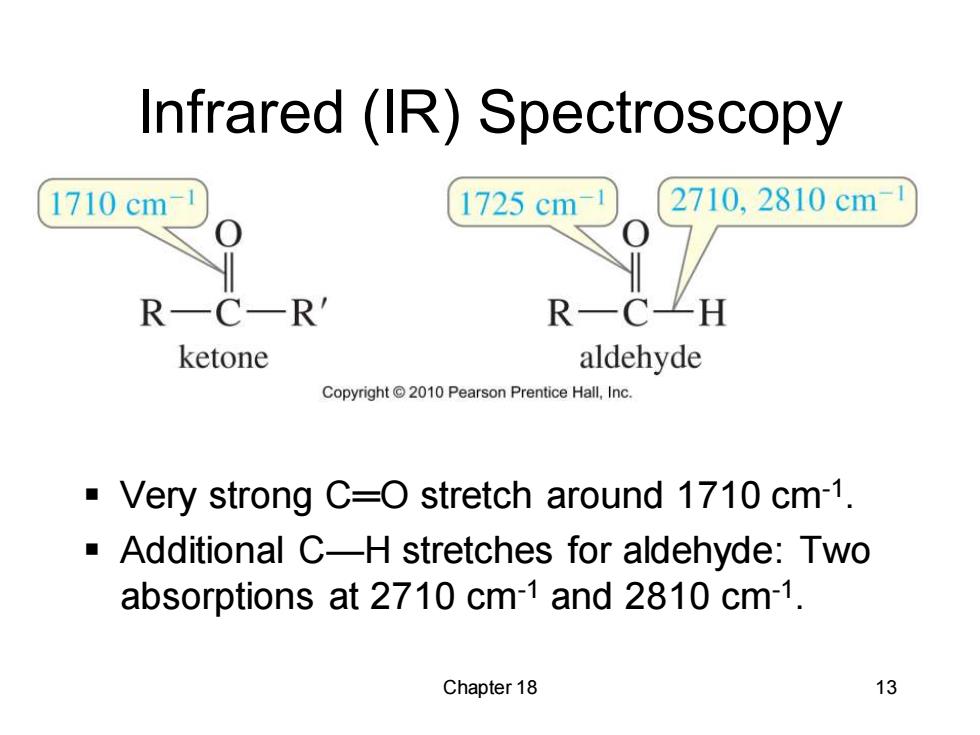
Infrared (IR)Spectroscopy 1710cm 1725cm 2710,2810cm R-C-R' R一CZH ketone aldehyde Copyright 2010 Pearson Prentice Hall,Inc. Very strong C=O stretch around 1710 cm-1. Additional C-H stretches for aldehyde:Two absorptions at 2710 cm-1 and 2810 cm-1. Chapter 18 13
Chapter 18 13 Infrared (IR) Spectroscopy ▪ Very strong C═O stretch around 1710 cm-1 . ▪ Additional C—H stretches for aldehyde: Two absorptions at 2710 cm-1 and 2810 cm-1
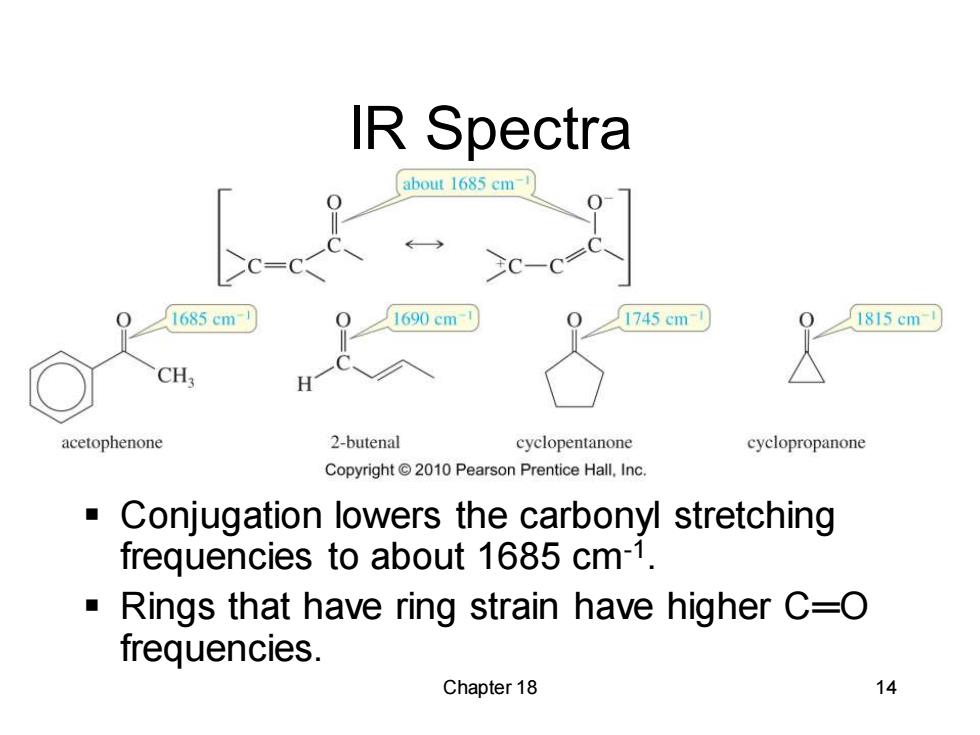
IR Spectra about 1685 cm 2c-cc. 1685cm 1690 cm 1745cm 1815cm CH. H acetophenone 2-butenal cyclopentanone cyclopropanone Copyright2010 Pearson Prentice Hall.Inc. Conjugation lowers the carbonyl stretching frequencies to about 1685 cm-1. Rings that have ring strain have higher C=O frequencies. Chapter 18 14
Chapter 18 14 IR Spectra ▪ Conjugation lowers the carbonyl stretching frequencies to about 1685 cm-1 . ▪ Rings that have ring strain have higher C═O frequencies
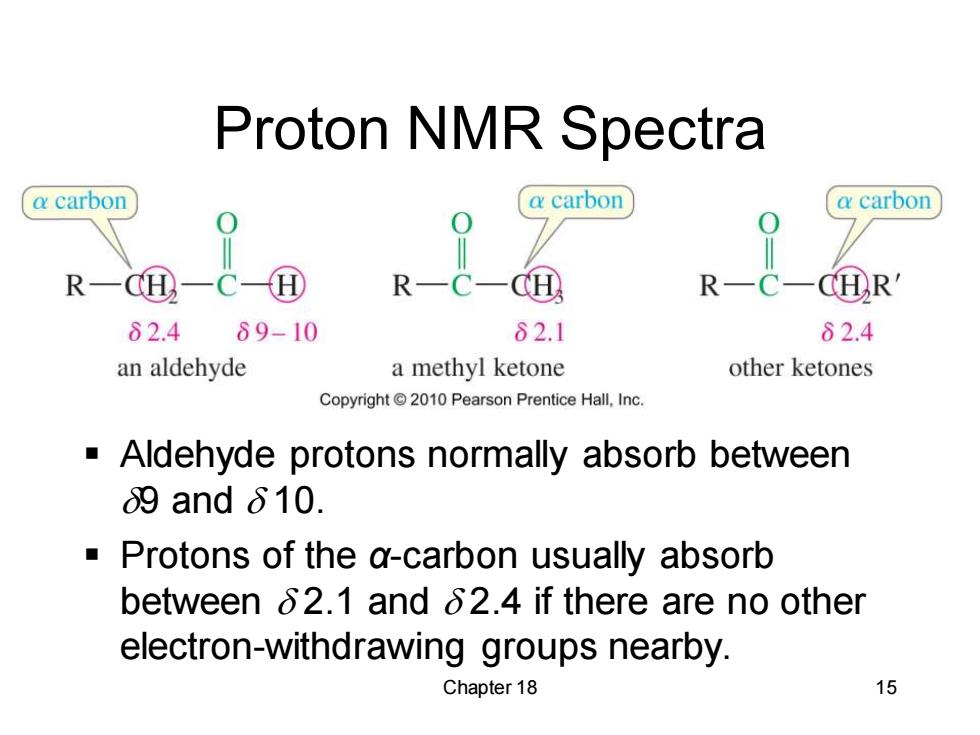
Proton NMR Spectra a carbon a carbon a carbon R R d① R CHR 82.4 89-10 82.1 82.4 an aldehyde a methyl ketone other ketones Copyright2010 Pearson Prentice Hall,Inc. Aldehyde protons normally absorb between 9and610. a Protons of the a-carbon usually absorb between 62.1 and 62.4 if there are no other electron-withdrawing groups nearby. Chapter 18 15
Chapter 18 15 Proton NMR Spectra ▪ Aldehyde protons normally absorb between d9 and d 10. ▪ Protons of the α-carbon usually absorb between d 2.1 and d 2.4 if there are no other electron-withdrawing groups nearby