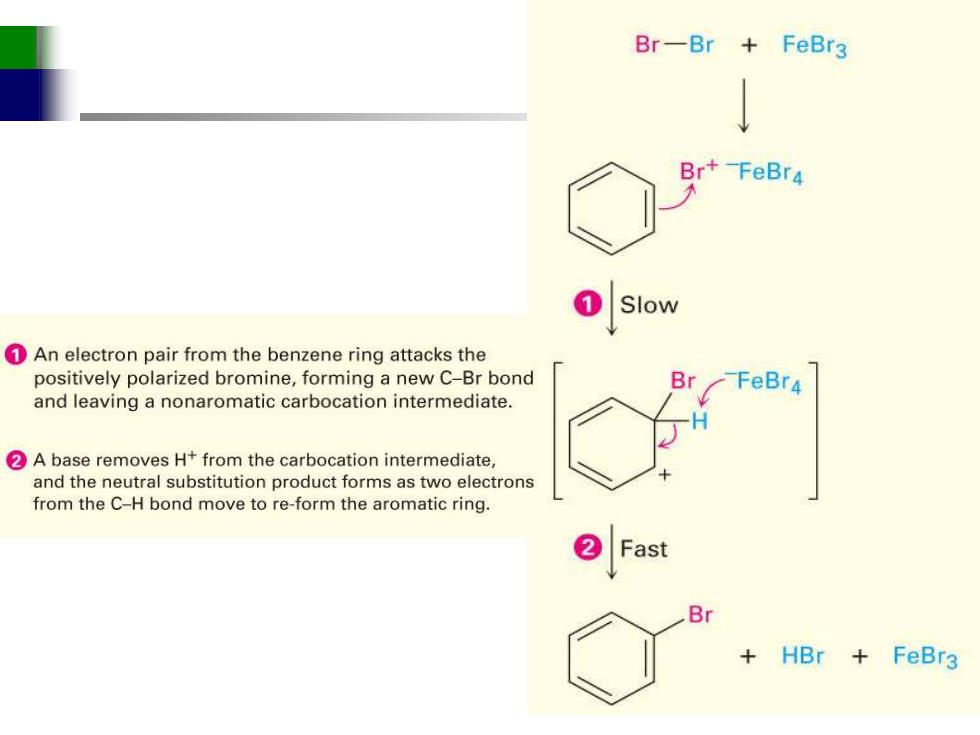
Br-Br+FeBr3 Br+-FeBr4 Slow 1An electron pair from the benzene ring attacks the positively polarized bromine,forming a new C-Br bond Br -FeBr4 and leaving a nonaromatic carbocation intermediate. 2A base removes H+from the carbocation intermediate, and the neutral substitution product forms as two electrons from the C-H bond move to re-form the aromatic ring. Fast Br +HBr+ FeBr3
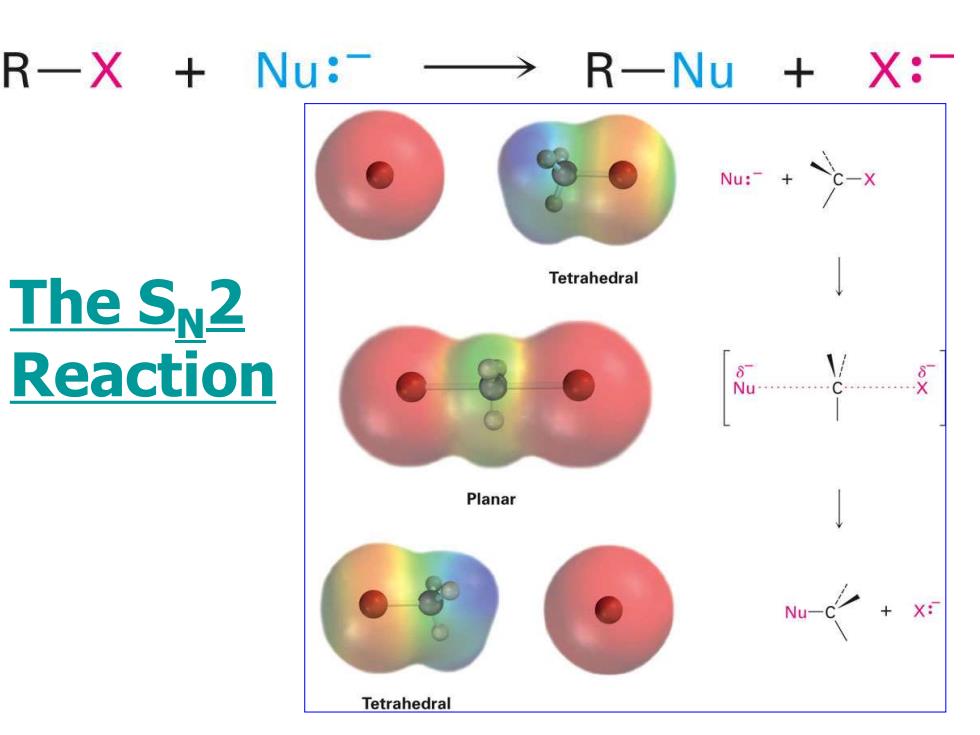
R一X+ Nu:-→ R一Nu+X:- Nu:-+ c-x Tetrahedral The SN2 Reaction Planar Nu-C X: Tetrahedral
The SN2 Reaction
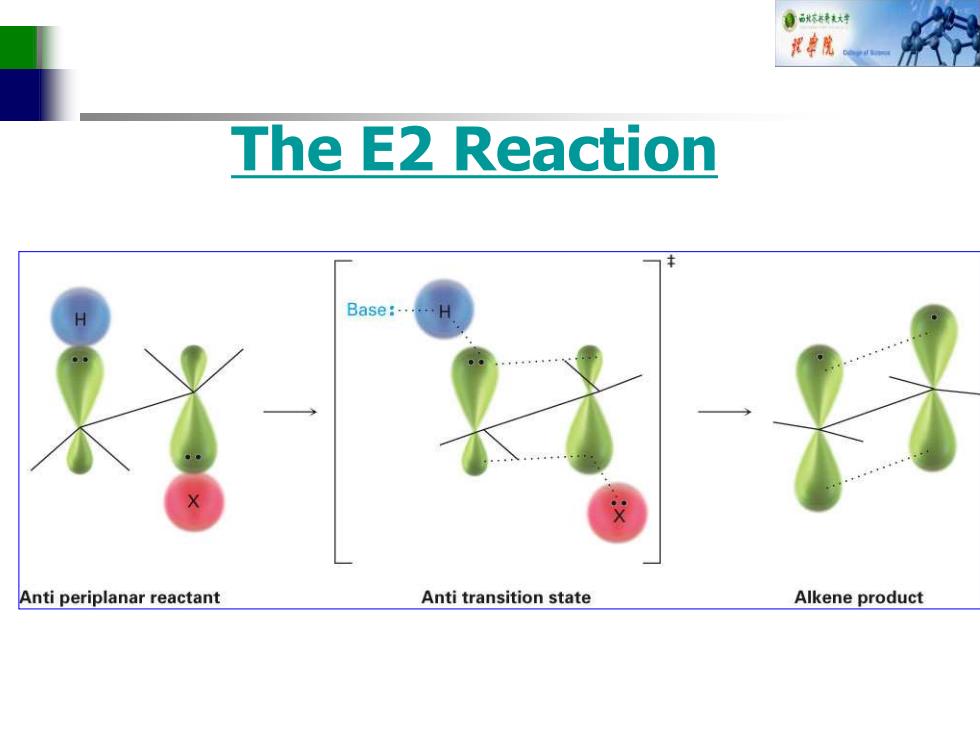
自秋转大好 花串院 The E2 Reaction Base:.. X Anti periplanar reactant Anti transition state Alkene product
The E2 Reaction

:H H3C 1An electron pair from the nucleophile attacks the electrophilic carbon of the Ketone carbonyl group,pushing an electron pair from the C=O bond onto oxygen and giving an alkoxide anion.The carbonyl carbon rehybridizes from sp2 to sp3. H3C ②Protonation of the alkoxide anion resulting Alkoxide ion from nucleophilic addition yields the ②Ho+ neutral alcohol addition product. :0H H20 H3C Alcohol 2007 Thomson Higher Education
自秋特大材 Sec 1 Classification Reactions can be classified as carbon-carbon bond formations construct complex molecules from simple starting materials In general,these reactions are the most difficult and temperamental to carry out. reactions are so important that they are named after the scientists who developed them (e.g.Grignard and Aldol reactions) Also classified according to the process or mechnism
Reactions can be classified as ◼ carbon-carbon bond formations ⚫ construct complex molecules from simple starting materials ⚫ In general, these reactions are the most difficult and temperamental to carry out. ⚫ reactions are so important that they are named after the scientists who developed them (e.g. Grignard and Aldol reactions) ❖Also classified according to the process or mechnism Sec 1 Classification