
HAc(aq)+H2O(D-H3O(aq)+Ac(aq) [HO]=+K[HA]...accurate expression 1 When both c and K are not too samll,ck>20 K the autoionization of H,O can be ignored,then: [HO]KTHA]=VKC-[H;O') H,O+KH,0]-c≈0H,01≈K,+40.K9 近似式 2 2 When cK 20 K,c is not too small,K is not too big,c/Ka 2500,the extent of inonizationof the acid can be ignored,[HA]C(HA),then: H,O]A]≈K simplest form 3 When cKo<20K9,c/K,≥500,then: [HO]≈[A]W+c
When cKaΘ ≥ 20 KwΘ, c is not too small, Ka is not too big, c/Ka ≥ 500 , the extent of inonizationof the acid can be ignored, [HA] ≈ C(HA) , then: When both c and Ka are not too samll,cKaΘ ≥ 20 KwΘ, the autoionization of H2O can be ignored,then: simplest form ≈ ≈ + − θ [H3O ] [A ] a cK θ a θ [H3O ] ≈[A ]≈ Kw +cK + − θ θ 3 wa [H O ] [HA] accurate expression K K + = + ⋅⋅⋅⋅⋅⋅⋅⋅⋅ 1 2 3 [H3O+] ≈ Kaθ 2 + 4 cKaθ - Kaθ 2 + − ≈ + + [H O ] [H O ] 0 θ 3 2 θ 3 a a K cK When cKaΘ < 20 KwΘ , c / K a ≥ 500,then: HAc(aq)+H2O(l) H3O+(aq)+Ac-(aq) 近似式 [H O ] [HA] ( [H O ]) 3 θa θ 3 a + + ≈ K = K c −

(continued) HAc(aq)+H2O(I)=H;O*(aq)+Ac (aq) Initial/mol-L-1 0.10 K2o(HAc)=1.8x10-5 When both c and K are not too samll,cK 20 K,the autoionization of H,O can be ignored,then: [H O']=K[HA]=K(c-[HOD) HO子+KHO]-cK=0 [H30]=1.3×10-3
Initial/mol·L-1 0.10 HAc(aq)+H2O(l) H3O+(aq)+Ac-(aq) [H O ] [HA] ( [H O ]) 3 θa θ 3 a + + = K = K c − + − = + + [H O ] [H O ] 0 θ 3 2 θ 3 a a K cK KaΘ(HAc) = 1.8 x 10-5 When both c and Ka are not too samll,cKaΘ ≥ 20 KwΘ, the autoionization of H2O can be ignored,then: [H3O+] = 1.3×10-3 (continued)

An alternative method: Since cKa>20 Kw,ionization of H2O can be ignored HAc(aq)+H2O(1)-H3O(aq)+Ac(aq) Initial/mol-L-1 0.10 0 Equi./mol-L-1 0.10-x X X K(HAc)=cH,0】[c(Ac〗 =1.8x10-5 Lc(HAc)] K(HAC)= x 0.10-x [H,O?+H,O]-cK=0 x=1.3×10-3
Initial/mol·L-1 0.10 0 0 Equi./mol·L-1 0.10-x xx x=1.3×10-3 HAc(aq)+H2O(l) H3O+(aq)+Ac-(aq) [ ] (HAc) (H O )] (Ac )] (HAc) 3 c [c [c + − K = a 0.10 x x (HAc) 2 − K = a = 1.8 x 10-5 + − = + + [H O ] [H O ] 0 θ 3 2 θ 3 a a ≡ K cK An alternative method: Since cKaΘ ≥ 20 KwΘ, ionization of H2O can be ignored

c(HO)=c(Ac)=1.3X10-3 molL-1 c(HAc)=(0.10-1.3×10-3)molL1≈0.10molL1 K9=c{(H3O)}{c(OH-)} c(0H-)=7.7×10-12molL1 pH=-lg{c(H,O*)}=2.89 percent ionizaton(a解离度 ionized concentration Q= -x100%=0-cex100% original concentration Co 1.3×103 a(HAc)= ×100%=1.3% 0.10
percent ionizaton ( a) 解离度 c(H 3 O +) = c(Ac -) = 1.3 ×10 - 3 mol·L-1 c(HAc)=(0.10 -1.3 ×10 - 3)mol·L-1 ≈0.10mol·L-1 c(OH -) =7.7 ×10 -12 mol·L-1 pH lg { (H O ) } 2.89 = − 3 = + c =c{(H 3 O + KW )}{ c(OH -)} 100 % 1.3 % 0.10 1.3 10 3 × = × = − α (HAc) α ionized concentration original concentration α = x 100% = c0 - ceq c0 x 100%
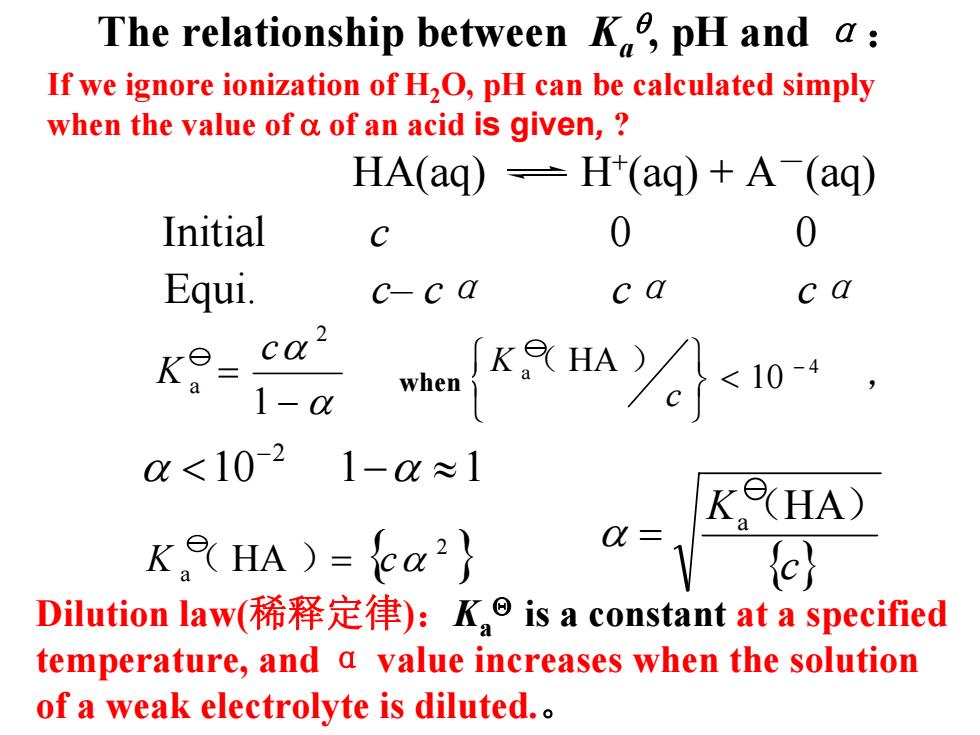
The relationship between K,pH and a: If we ignore ionization of H2O,pH can be calculated simply when the value of a of an acid is given, HA(aq)=H(aq)+A(aq) Initial C 0 Equi. c-ca c a c a K9- ca2 1-a KHA合10, when 0<10-2 1-0≈1 KHA) KHA )=ca2 a=) Dilution law(稀释定律):K is a constant at a specified temperature,and a value increases when the solution of a weak electrolyte is diluted
The relationship between Ka θ , pH and α : HA(aq) H +(aq) + A -(aq) Equi. c– c α c α c α Initial c 0 0 Dilution law(稀释定律 ) : Ka Θ is a constant at a specified temperature, and α value increases when the solution of a weak electrolyte is diluted. 。 10 1 1 2 < − ≈ − α α α α − = 1 2 a c K 时, ( ) 当 10 HA 4 a − < ⎭ ⎬ ⎫ ⎩ ⎨ ⎧ c K { } 2 K a ( HA ) = c α { }c K a (HA ) α = If we ignore ionization of H 2O, pH can be calculated simply when the value of α of an acid is given , ? when