
浓差极化:由于电解过程中电极表面附近溶液 的浓度和主体溶液浓度产生了差别所引起的。 pa>Pa平) pe<Pc平) 大小与搅拌、电流密度大小有关 减少浓差极化方法: ①增大电极面积 ②减少电流密度 ③提高溶液温度 ④机械搅拌
浓差极化:由于电解过程中电极表面附近溶液 的浓度和主体溶液浓度产生了差别所引起的。 φa ﹥φa(平) φc ﹤φc(平) 大小与搅拌、电流密度大小有关 减少浓差极化方法: ①增大电极面积 ②减少电流密度 ③提高溶液温度 ④机械搅拌
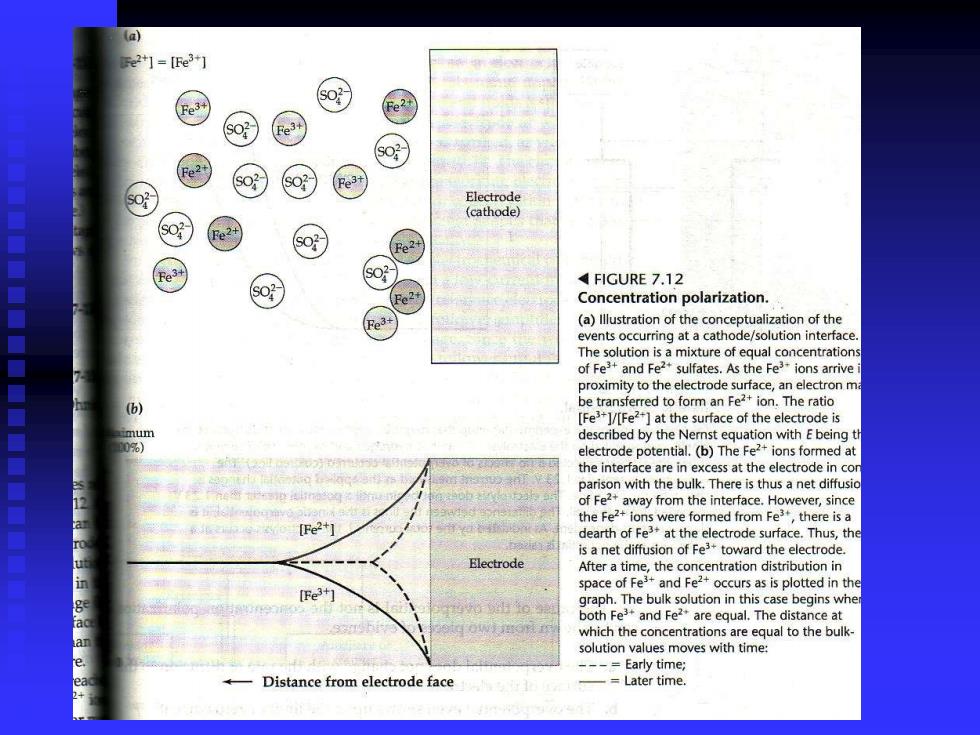
门=Fe31 3+ SO? Electrode (cathode) e 4FIGURE 7.12 Fo- Concentration polarization. Fe3 (a)Illustration of the conceptualization of the events occurring at a cathode/solution interface The solution is a mixture of equal concentrations of Feand Fesulfates.As the Feions arrive proximity to the electrode surface,an electron m be transferred to form an Fe2+ion.The ratio IFe3+1/[Fe2+]at the surface of the electrode is described by the Nemnst equation with E being th electrode potential.(b)The Fef ions formed at the interface are in excess at the electrode in cor parison with the bulk.There is thus a net diffusio of Fe2+away from the interface.However,since the Fe2+ions were formed from Fe,there is a dearth of Fe3 at the electrode surface.Thus,the is a net diffusion of Fe toward the electrode. Electrode After a time,the concentration distribution in space of Fe3+and Fe2+occurs as is plotted in the [Fe3+ graph.The bulk solution in this case begins whe both Fe*and Fe are equal.The distance at which the concentrations are equal to the bulk solution values moves with time: -Early time; Distance from electrode face Later time