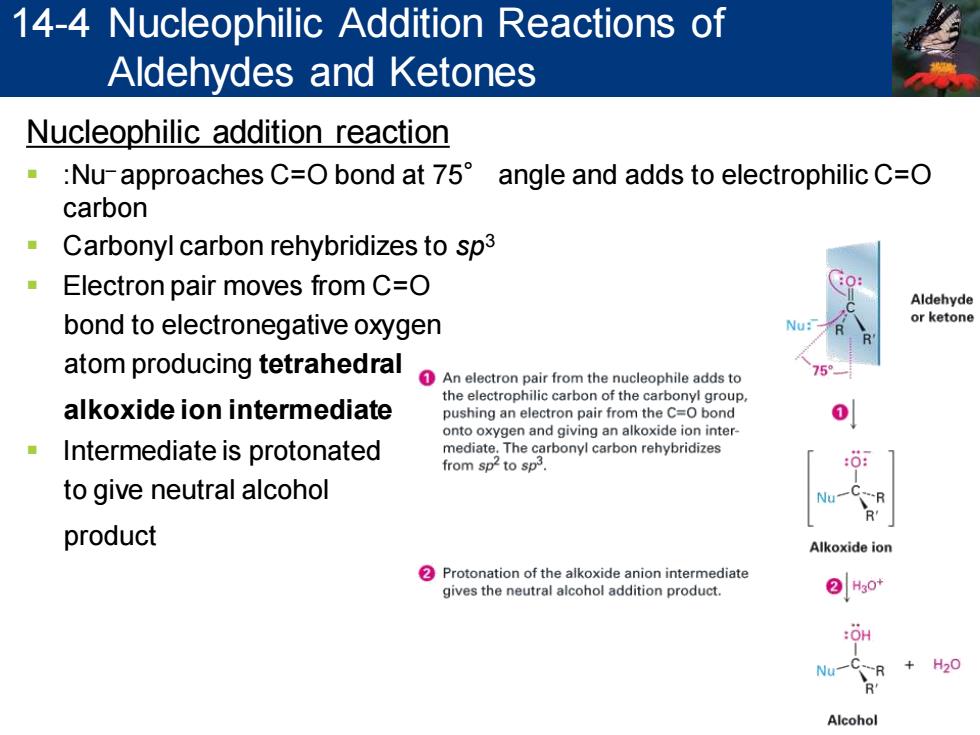
14-4 Nucleophilic Addition Reactions of Aldehydes and Ketones Nucleophilic addition reaction Nu-approaches C=O bond at 75 angle and adds to electrophilic C=O carbon Carbonyl carbon rehybridizes to sp3 Electron pair moves from C=O Aldehyde bond to electronegative oxygen or ketone atom producing tetrahedral An electron pair from the nucleophile adds to alkoxide ion intermediate the electrophilic carbon of the carbonyl group pushing an electron pair from the C=O bond 0 onto oxygen and giving an alkoxide ion inter Intermediate is protonated mediate.The carbonyl carbon rehybridizes from sp to sp. to give neutral alcohol Nu product Alkoxide ion 2 Protonation of the alkoxide anion intermediate gives the neutral alcohol addition product. ②Hg0 :OH Nu一 R H20 Alcohol
Nucleophilic addition reaction ▪ :Nu– approaches C=O bond at 75° angle and adds to electrophilic C=O carbon ▪ Carbonyl carbon rehybridizes to sp3 ▪ Electron pair moves from C=O bond to electronegative oxygen atom producing tetrahedral alkoxide ion intermediate ▪ Intermediate is protonated to give neutral alcohol product 14-4 Nucleophilic Addition Reactions of Aldehydes and Ketones

Nucleophilic Addition Reactions of Aldehydes and Ketones Nucleophiles can be either negatively charged (Nu )or neutral (:Nu) If neutral,the nucleophile usually carries a hydrogen atom that can be subsequently eliminated HO:-(hydroxide ion) H:(hydride ion) Some negatively charged nucleophiles R3C:(a carbanion) RO:(an alkoxide ion) N=C:(cyanide ion) HOH(water) ROH(an alcohol) Some neutral nucleophiles H3N:(ammonia) RNH2(an amine)
Nucleophiles can be either negatively charged (:Nu- ) or neutral (:Nu) ▪ If neutral, the nucleophile usually carries a hydrogen atom that can be subsequently eliminated Nucleophilic Addition Reactions of Aldehydes and Ketones
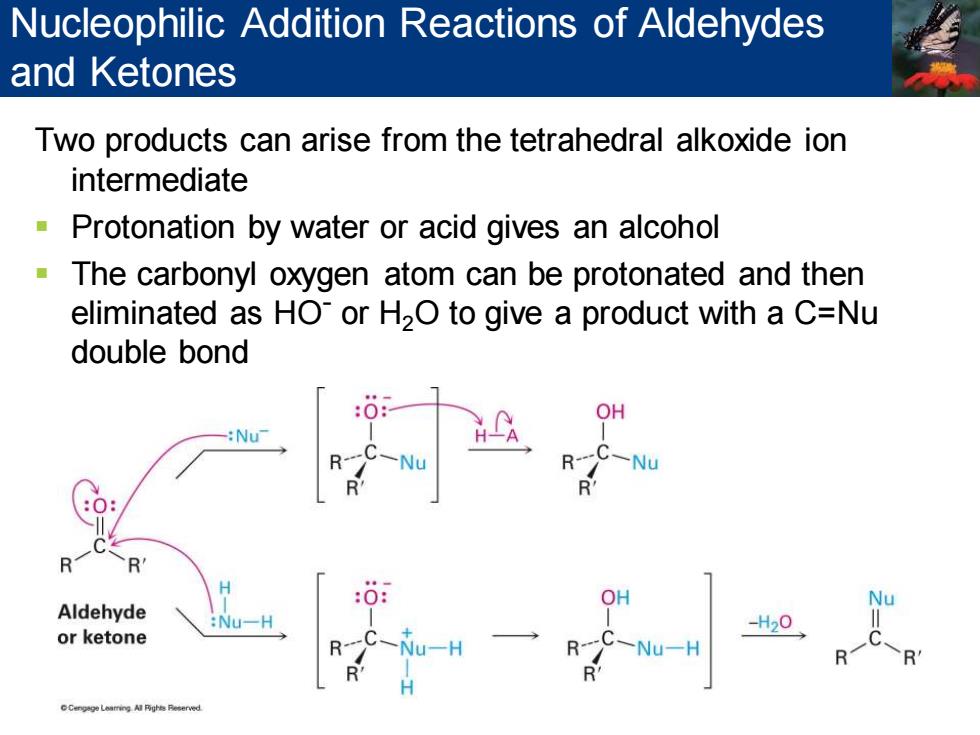
Nucleophilic Addition Reactions of Aldehydes and Ketones Two products can arise from the tetrahedral alkoxide ion intermediate Protonation by water or acid gives an alcohol The carbonyl oxygen atom can be protonated and then eliminated as HO or H2O to give a product with a C=Nu double bond OH :Nu -Nu C一Nu H :0 Aldehyde Nu一H -H20 or ketone Nu-H -Nu-H
Two products can arise from the tetrahedral alkoxide ion intermediate ▪ Protonation by water or acid gives an alcohol ▪ The carbonyl oxygen atom can be protonated and then eliminated as HO- or H2O to give a product with a C=Nu double bond Nucleophilic Addition Reactions of Aldehydes and Ketones
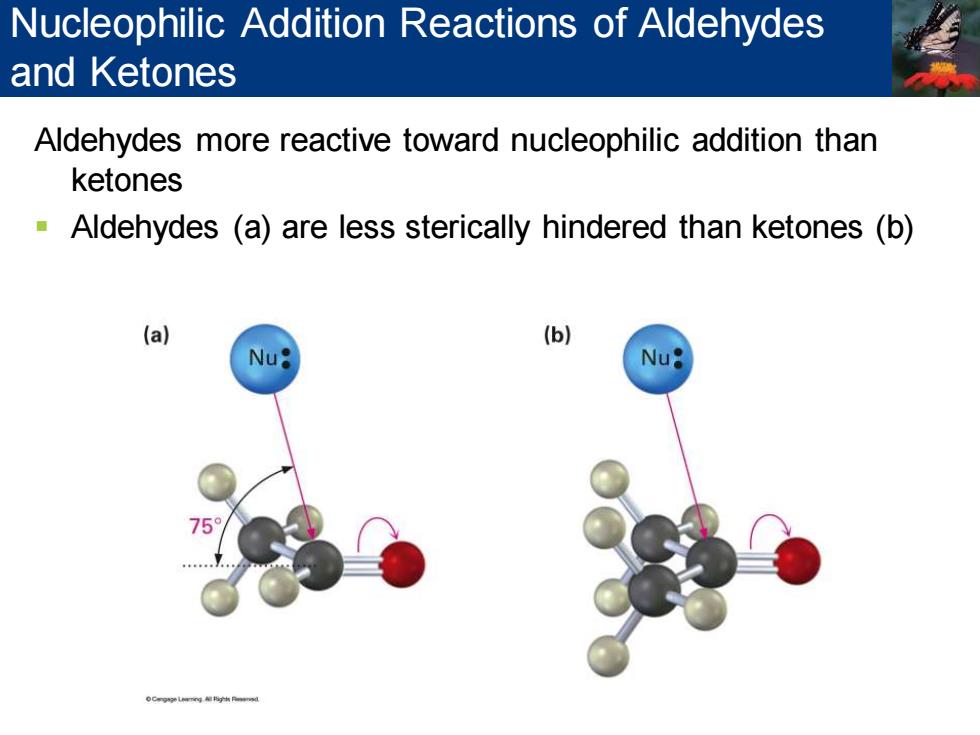
Nucleophilic Addition Reactions of Aldehydes and Ketones Aldehydes more reactive toward nucleophilic addition than ketones Aldehydes (a)are less sterically hindered than ketones (b) (a) (b) Nu
Aldehydes more reactive toward nucleophilic addition than ketones ▪ Aldehydes (a) are less sterically hindered than ketones (b) Nucleophilic Addition Reactions of Aldehydes and Ketones
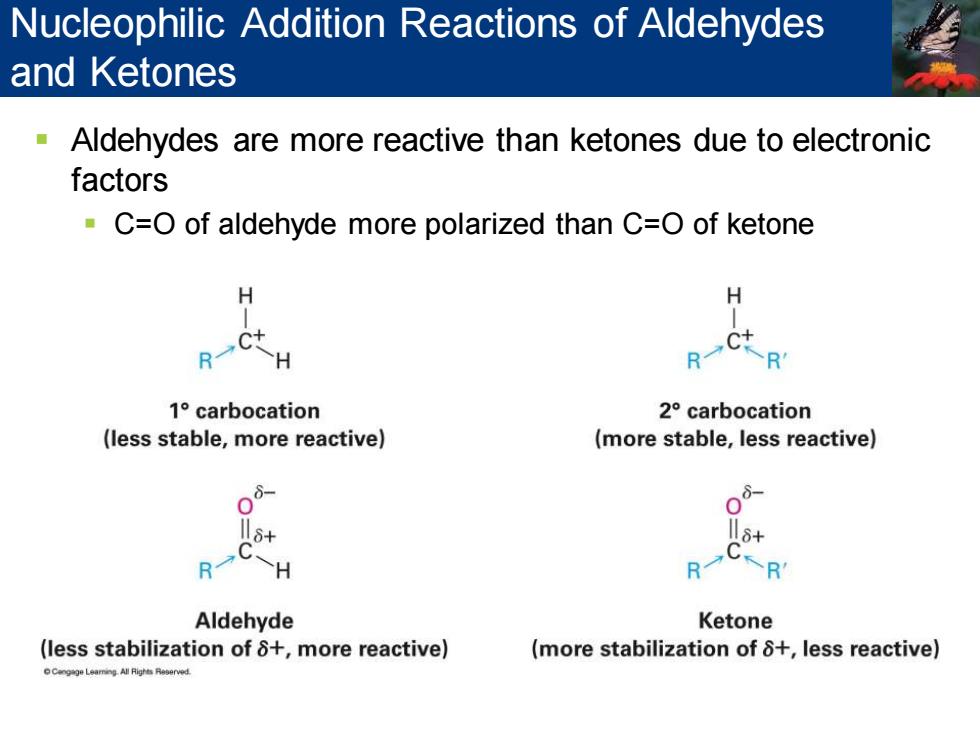
Nucleophilic Addition Reactions of Aldehydes and Ketones Aldehydes are more reactive than ketones due to electronic factors C=O of aldehyde more polarized than C=O of ketone H H R H C+-R 1°carbocation 2°carbocation (less stable,more reactive) (more stable,less reactive) 8+ >H KR Aldehyde Ketone (less stabilization of 8+,more reactive) (more stabilization of 8+,less reactive)
▪ Aldehydes are more reactive than ketones due to electronic factors ▪ C=O of aldehyde more polarized than C=O of ketone Nucleophilic Addition Reactions of Aldehydes and Ketones