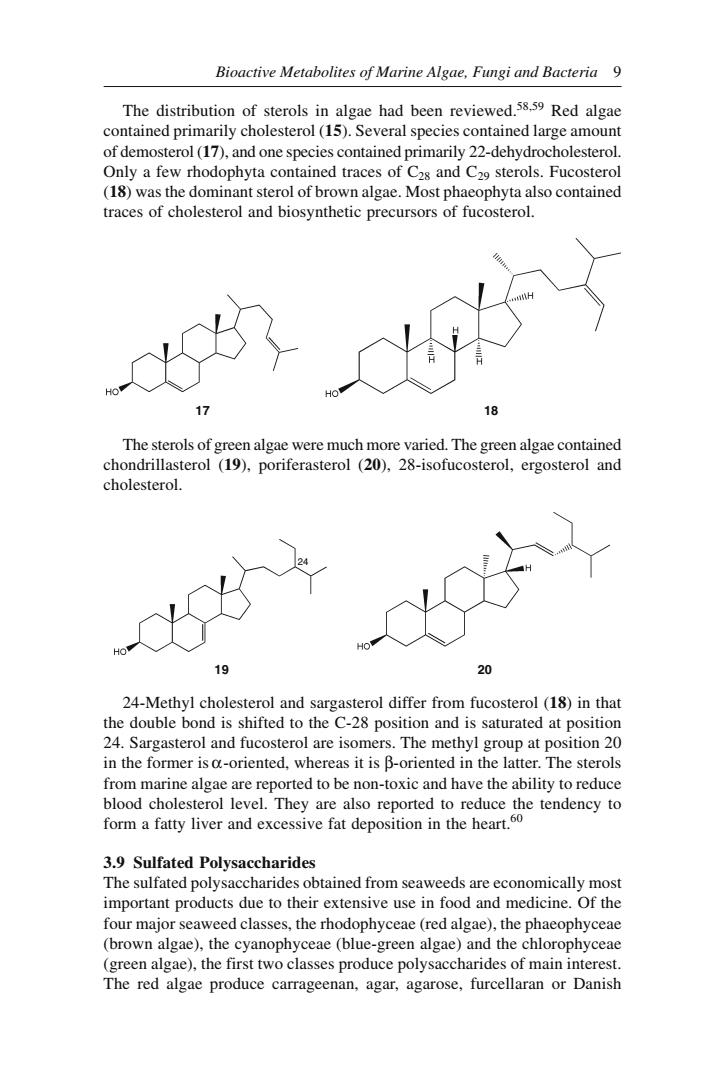
Bioactive Metabolites of Marine Algae,Fungi and Bacteria 9 The distribution of sterols in algae had been reviewed Red algae contained primarily cholesterol(15).Several species contained large amount of demosterol(17),and one species contained primarily 22-dehydrocholesterol. Only a few rhodophyta contained traces of C2s and C29 sterols.Fucosterol (18)was the dominant sterol of brown algae.Most phaeophyta also contained traces of cholesterol and biosynthetic precursors of fucosterol. The sterols of n alga vere much more varied.The green algae contained ondrillas terol (19),poriferasterol(20),28-isofucosterol,ergosterol an cholesterol. 19 24-Methyl cholesterol and sargasterol differ from fucosterol (18)in that bond is shifted to the C-28 in the former is a-oriented,whereas it is B-oriented in the latter.The sterols from marine algae are reported to be non-toxic and have the ability to reduce 3.9 Sulfated Polysaccharides The sulfated polysaccharides obtained from seaweeds are economically most important products due to their extensive use in food and medicine.Of the four major seaweed classes,the rhodophyceae(red algae),the phaeophyceae (brown agae)the yanophyceae(algae)and the chlorophyceae The reage produce caragecnan.agrose,furcellar or
Bioactive Metabolites of Marine Algae, Fungi and Bacteria 9 24-Methyl cholesterol and sargasterol differ from fucosterol (18) in that the double bond is shifted to the C-28 position and is saturated at position 24. Sargasterol and fucosterol are isomers. The methyl group at position 20 in the former is α-oriented, whereas it is β-oriented in the latter. The sterols from marine algae are reported to be non-toxic and have the ability to reduce blood cholesterol level. They are also reported to reduce the tendency to form a fatty liver and excessive fat deposition in the heart.60 3.9 Sulfated Polysaccharides The sulfated polysaccharides obtained from seaweeds are economically most important products due to their extensive use in food and medicine. Of the four major seaweed classes, the rhodophyceae (red algae), the phaeophyceae (brown algae), the cyanophyceae (blue-green algae) and the chlorophyceae (green algae), the first two classes produce polysaccharides of main interest. The red algae produce carrageenan, agar, agarose, furcellaran or Danish The distribution of sterols in algae had been reviewed.58,59 Red algae contained primarily cholesterol (15). Several species contained large amount of demosterol (17), and one species contained primarily 22-dehydrocholesterol. Only a few rhodophyta contained traces of C28 and C29 sterols. Fucosterol (18) was the dominant sterol of brown algae. Most phaeophyta also contained traces of cholesterol and biosynthetic precursors of fucosterol. The sterols of green algae were much more varied. The green algae contained chondrillasterol (19), poriferasterol (20), 28-isofucosterol, ergosterol and cholesterol. 17 18 19 20 24

10 Bioactive Marine Natural Products agar.Alginic acid is obtained from brown algae.The use of seaweed extracts in food and medicine is reviewed.61 Carrageenan are produced by species of Chondrus,Eucheuma,Gigartina and Iridea.There are different views on the aride compri ng D-galactose unit and derivatives linked alternatively a(13)and B(1-4).The L K,A and u and other carrageenans represent variations of this primary and general form in which the galactose units are sulfated in a definite pattern and/or are the validit of the above simplified structural approach to carrageenan.These workers believe that carrageenan is a continum of potassium precipitable material of continuously variable structural form.The ester sulphate groups are distributed randomly on all available hydroxyl groups in K,in support of this hypothesis.The chemical structure of k and carrageenans are still a matter of discussion.Carrageenan is precipitated from dilute solution with potassium ions. and is believed to consist primarily of alternating anhydrogalactose and sulphated galactose inits linked o 13 and B 14.A Carrageenan contains little anhydrogalactose. It consists chiefly of mono-and disulphate galactose units with,perhaps,the same alternating 13 and l4-linkages.Both kand A carrageenans are reported to be strong an The latter is more potent than the former.In gen eral they behave as typical carbohydrate antigens Carrageenan is also reporte to stimulate the growth of otve tissues Chondrus crispus and Gelidium cartilagineum,the well-known sources of carrageenan and agar,respectively,had been found to possess antiviral properties attributed to the galactan units in the polysaccharides of both.The antiviral activity had been t influ enza B and mump inembryonated eggs even afte inocubat on.Carrageenan was also found as anticoagulant and antithrombic agent.The use of carrageenan in ulcer therapy had been studied intensively.It was thought at first that the polysaccharide inhibits the activity of pepsin and that its action in preventing ulers was due to this property However.subsequent studies revealed that plays a much more active r thefound that in viv,pepsn was not enzyme inhibition.In ed by carrageenan. The polysaccharide reacts with the mucoid lining of the stomach and gives a protective layer through which pepsin and acid have difficulty in passing. The treatment of gastric and duodenal ulcers by carrageenan was enjoying popularity in France and Great Britain.In many cases of ulce carrageenan proved an effective cure.6 Alginic Acid This polysaccharide is obtained from the brown seaweeds,especially from species of Fucus and Macrocystis.Chemically alginic acid(21)is made up of two monomers,the D-mannuronic acid and L-guluronic acid.Both these
10 Bioactive Marine Natural Products agar. Alginic acid is obtained from brown algae. The use of seaweed extracts in food and medicine is reviewed.61 Carrageenan are produced by species of Chondrus, Eucheuma, Gigartina and Iridea. There are different views on the structure of red seaweed polysaccharides.62 It is generally suggested that carrageenans be defined as a polysaccharide comprising D-galactose units and derivatives linked alternatively α (1→ 3) and β (1→ 4). The ι, κ, λ and µ and other carrageenans represent variations of this primary and general form in which the galactose units are sulfated in a definite pattern and/or are present in the 3,6-anhydro form expressed generally as an A-B-A polymer. Pernas et al63 however, do not agree on the validity of the above simplified structural approach to carrageenan. These workers believe that carrageenan is a continum of potassium precipitable material of continuously variable structural form. The ester sulphate groups are distributed randomly on all available hydroxyl groups in κ, in support of this hypothesis. The chemical structure of κ and λ carrageenans are still a matter of discussion. κ Carrageenan is precipitated from dilute solution with potassium ions, and is believed to consist primarily of alternating anhydrogalactose and sulphated galactose units linked α 1,3 and β 1,4. λ Carrageenan contains little anhydrogalactose. It consists chiefly of mono- and disulphate galactose units with, perhaps, the same alternating 1,3 and 1,4-linkages. Both κ and λ carrageenans are reported to be strong antigens.64 The latter is more potent than the former. In general, they behave as typical carbohydrate antigens. λ Carrageenan is also reported to stimulate the growth of connective tissues.64 Chondrus crispus and Gelidium cartilagineum, the well-known sources of carrageenan and agar, respectively, had been found to possess antiviral properties attributed to the galactan units in the polysaccharides of both. The specific antiviral activity had been shown against influenza B and mumps virus in embryonated eggs even after 24 h inocubation. Carrageenan was also found as anticoagulant and antithrombic agent. The use of carrageenan in ulcer therapy had been studied intensively. It was thought at first that the polysaccharide inhibits the activity of pepsin and that its action in preventing ulcers was due to this property.65 However, subsequent studies revealed that the polysaccharide plays a much more active role than enzyme inhibition. In fact, it was found that in vivo, pepsin was not inhibited by carrageenan. The polysaccharide reacts with the mucoid lining of the stomach and gives a protective layer through which pepsin and acid have difficulty in passing. The treatment of gastric and duodenal ulcers by carrageenan was enjoying considerable popularity in France and Great Britain. In many cases of ulcer carrageenan proved an effective cure.66 Alginic Acid This polysaccharide is obtained from the brown seaweeds, especially from species of Fucus and Macrocystis. Chemically alginic acid (21) is made up of two monomers, the D-mannuronic acid and L-guluronic acid. Both these
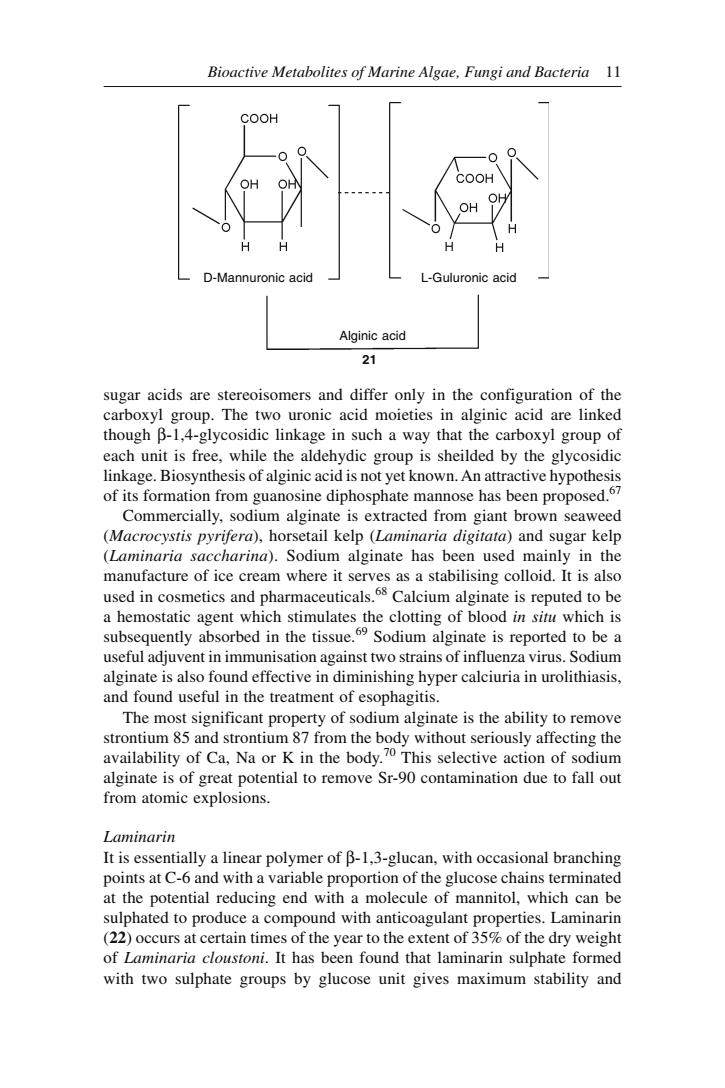
Bioactive Metabolites of Marine Algae,Fungi and Bacteria 11 CooH L-Guluronic Alginic acid 21 sugar acids are stereoisomers and differ only in the configuration of the arboxyl grou each unit is free.while the aldehydic group is sheilded by the glycosidi linkage.Biosynthesis of alginic acid is not yet known.An attractive hypothesis of its formation from guanosine diphosphate mannose has been proposed. Commercially,sodium alginate is extracted from giant brown seaweed (Macrocystis pyriferd),horsetail kelp()and sugar kelp aria sac charina).Sodium alginate has manufacture of ice cream where it serves as a stabilising colloid.It isa m used in cosmetics and pharmaceuticals.Calcium alginate is reputed to be a hemostatic agent which stimulates the clotting of blood in situ which is subsequently absorbed in the tissue.Sodium alginate is reported to be a seful adiuy vent in immunisation against two strains of influenza virus.Sodium alginate is also found effective in diminishing hyper calciuria in urolithiasis. and found useful in the treatment of esophagitis. The most significant property of sodium alginate is the ability to remove strontium 85 and strontium 87 from the body without seriously affecting the availability of Ca,Na or K in the body.70 This selective action of sodium inate is of ential to remove Sr-90 ion due to fall ou from atomic explosions Laminarin It is essentially a linear polymer of B-1.3-glucan,with occasional branching points at c-6 and with a variable proportion of the glucose chains terminated at the potential reducing end with mannito which nd with anticoagulant properties Lamina erntimes of the year to the extent of 3 of the dryweiht of Laminaria cloustoni.It has been found that laminarin sulphate formed with two sulphate groups by glucose unit gives maximum stability and
Bioactive Metabolites of Marine Algae, Fungi and Bacteria 11 sugar acids are stereoisomers and differ only in the configuration of the carboxyl group. The two uronic acid moieties in alginic acid are linked though β-1,4-glycosidic linkage in such a way that the carboxyl group of each unit is free, while the aldehydic group is sheilded by the glycosidic linkage. Biosynthesis of alginic acid is not yet known. An attractive hypothesis of its formation from guanosine diphosphate mannose has been proposed.67 Commercially, sodium alginate is extracted from giant brown seaweed (Macrocystis pyrifera), horsetail kelp (Laminaria digitata) and sugar kelp (Laminaria saccharina). Sodium alginate has been used mainly in the manufacture of ice cream where it serves as a stabilising colloid. It is also used in cosmetics and pharmaceuticals.68 Calcium alginate is reputed to be a hemostatic agent which stimulates the clotting of blood in situ which is subsequently absorbed in the tissue.69 Sodium alginate is reported to be a useful adjuvent in immunisation against two strains of influenza virus. Sodium alginate is also found effective in diminishing hyper calciuria in urolithiasis, and found useful in the treatment of esophagitis. The most significant property of sodium alginate is the ability to remove strontium 85 and strontium 87 from the body without seriously affecting the availability of Ca, Na or K in the body.70 This selective action of sodium alginate is of great potential to remove Sr-90 contamination due to fall out from atomic explosions. Laminarin It is essentially a linear polymer of β-1,3-glucan, with occasional branching points at C-6 and with a variable proportion of the glucose chains terminated at the potential reducing end with a molecule of mannitol, which can be sulphated to produce a compound with anticoagulant properties. Laminarin (22) occurs at certain times of the year to the extent of 35% of the dry weight of Laminaria cloustoni. It has been found that laminarin sulphate formed with two sulphate groups by glucose unit gives maximum stability and D-Mannuronic acid L-Guluronic acid Alginic acid 21
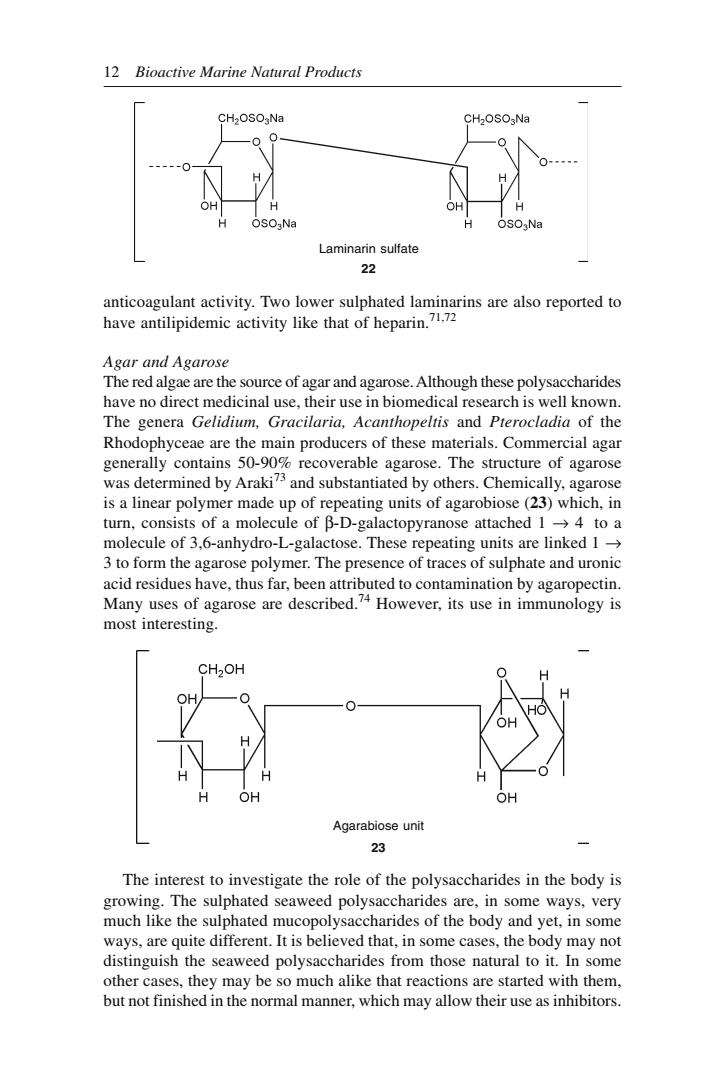
12 Bioactive Marine Natural Products CH2OSO,Na Laminarin sulfate 22 anicoaeulataciviy,Teoloerulphaiedhmiagrimsacalorepoiedo have antilipidemic activity like that of heparin. Agar and Agarose The red algae are the source of agar and agarose.Although these polysaccharides have no direct medicinal use,their use in biomedical research is well known. The ge era Gelidim.Gracilaria,Acanthopeltis and Prerocladia ofthe Rhodophyceae are the main producers of these materials.Commercial agar generally contains 50-90%recoverable agarose.The structure of agarose was determined by Araki and substantiated by others.Chemically,agarose is a linear polymer made up of repeating units of agarobiose(23)which,in turn,consists of a molecule of B-D-galactopyranose attached 14 to a its are linked 1 acid residues have,thus far,been attributed to contamination by agaropectin. Many uses of agarose are described.?4 However,its use in immunology is most interesting. CH2OH OH Agarabiose unit The interest to investigate the role of the polysaccharides in the body is growing.The sulphated seaweed polysaccharides are,in some ways,very much like the sulphated mucopolysaccharides of the body and yet.in some me cases,the bodv may no rides from those natural to it.In som other cases,they may be so much alike that reactions are started with them, but not finished in the normal manner,which may allow their use as inhibitors
12 Bioactive Marine Natural Products anticoagulant activity. Two lower sulphated laminarins are also reported to have antilipidemic activity like that of heparin.71,72 Agar and Agarose The red algae are the source of agar and agarose. Although these polysaccharides have no direct medicinal use, their use in biomedical research is well known. The genera Gelidium, Gracilaria, Acanthopeltis and Pterocladia of the Rhodophyceae are the main producers of these materials. Commercial agar generally contains 50-90% recoverable agarose. The structure of agarose was determined by Araki73 and substantiated by others. Chemically, agarose is a linear polymer made up of repeating units of agarobiose (23) which, in turn, consists of a molecule of β-D-galactopyranose attached 1 → 4 to a molecule of 3,6-anhydro-L-galactose. These repeating units are linked 1 → 3 to form the agarose polymer. The presence of traces of sulphate and uronic acid residues have, thus far, been attributed to contamination by agaropectin. Many uses of agarose are described.74 However, its use in immunology is most interesting. The interest to investigate the role of the polysaccharides in the body is growing. The sulphated seaweed polysaccharides are, in some ways, very much like the sulphated mucopolysaccharides of the body and yet, in some ways, are quite different. It is believed that, in some cases, the body may not distinguish the seaweed polysaccharides from those natural to it. In some other cases, they may be so much alike that reactions are started with them, but not finished in the normal manner, which may allow their use as inhibitors. Agarabiose unit 23 Laminarin sulfate 22
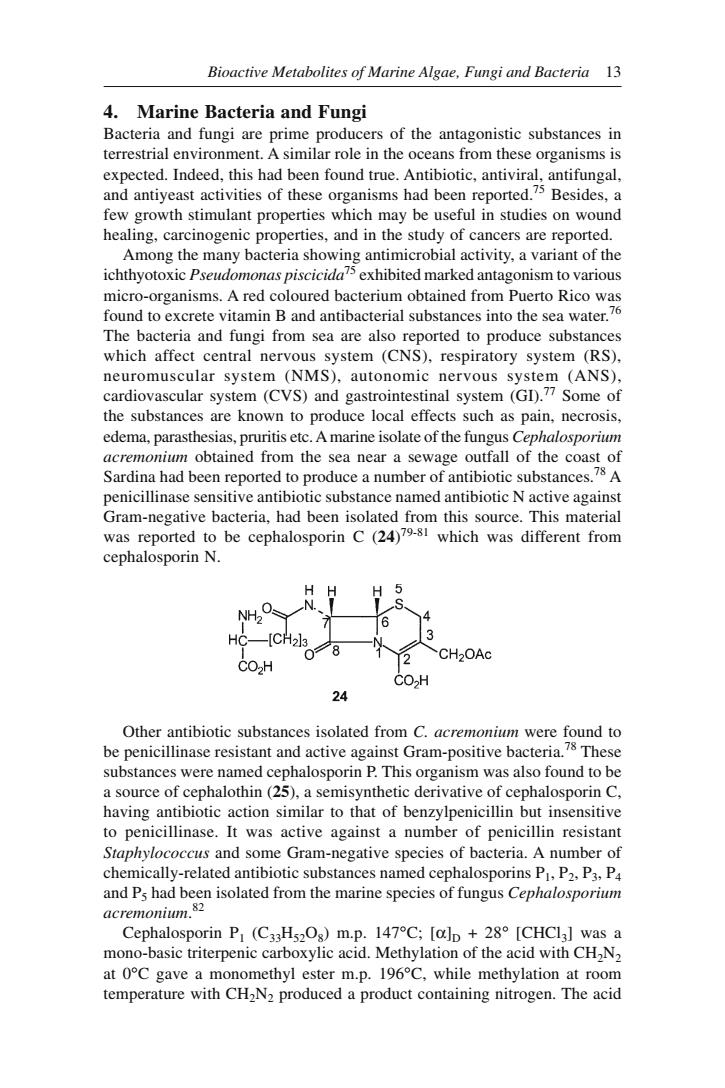
Bioactive Metabolites of Marine Algae,Fungi and Bacteria 13 4.Marine Bacteria and Fungi Bacteria and fungi are prime producers of the antagonistic substances in terrestrial environment.A similar role in the oceans from these organisms is expected.Indeed,this had been found true.Antibiotic,antiviral,antifungal. few growth stimulant properties which may be useful in studies on wound healing,carcinogenic properties,and in the study of cancers are reported. Among the many bacteria showing antimicrobial activity,a variant of the ichthyotoxic Pseudomonas piscicida75 exhibited marked antagonism to various micr vitamin B and antibacterial substar The bacteria and fungi from sea are also reported to produce substances which affect central nervous system (CNS).respiratory system (RS). neuromuscular system (NMS),autonomic nervous system (ANS). cardiovascular system (CVS)and gastrointestinal system (GD.Some of the substances are known to produce local effects such as pain,necrosis. edema.parasthesias,pruritis etc.Amarine isolate of the fungus Cephalosporm acremonium obtained from the sea near a sewage outfall of the coast of Sardina had been reported to produce a number of antibiotic substances.A penicillinase sensitive antibiotic substance named antibiotic N active against Gam-ncivbaceore.This maicrial reported to be was different from cephalosporin N NH C 24 Other antibiotic substances substances were named cephalosporin P.This organism was also found to be a source of cephalothin(25),a semisynthetic derivative of cephalosporin C. having antibiotic action similar to that of benzylpenicillin but insensitive to penicillinase.It was active against a number of penicillin resistant occus and some Gram-negative species of bacteria.A nu chem ally-related antibiotic substances named cephalosporins P.P2.P3.P4 n isolated from the marine species of fungus Cephalosporm Cephalosporin P (C3Hs,Os)m.p.147C:[aln +28 [CHCIa]was a mono-basic triterpenic carboxylic acid.Methylation of the acid with CHN at OC gave a mor omethyl ester m.p.196C.while methylation at room temperature with CH2N2 produced a product containing nitrogen.The acid
Bioactive Metabolites of Marine Algae, Fungi and Bacteria 13 4. Marine Bacteria and Fungi Bacteria and fungi are prime producers of the antagonistic substances in terrestrial environment. A similar role in the oceans from these organisms is expected. Indeed, this had been found true. Antibiotic, antiviral, antifungal, and antiyeast activities of these organisms had been reported.75 Besides, a few growth stimulant properties which may be useful in studies on wound healing, carcinogenic properties, and in the study of cancers are reported. Among the many bacteria showing antimicrobial activity, a variant of the ichthyotoxic Pseudomonas piscicida75 exhibited marked antagonism to various micro-organisms. A red coloured bacterium obtained from Puerto Rico was found to excrete vitamin B and antibacterial substances into the sea water.76 The bacteria and fungi from sea are also reported to produce substances which affect central nervous system (CNS), respiratory system (RS), neuromuscular system (NMS), autonomic nervous system (ANS), cardiovascular system (CVS) and gastrointestinal system (GI).77 Some of the substances are known to produce local effects such as pain, necrosis, edema, parasthesias, pruritis etc. A marine isolate of the fungus Cephalosporium acremonium obtained from the sea near a sewage outfall of the coast of Sardina had been reported to produce a number of antibiotic substances.78 A penicillinase sensitive antibiotic substance named antibiotic N active against Gram-negative bacteria, had been isolated from this source. This material was reported to be cephalosporin C (24) 79-81 which was different from cephalosporin N. Other antibiotic substances isolated from C. acremonium were found to be penicillinase resistant and active against Gram-positive bacteria.78 These substances were named cephalosporin P. This organism was also found to be a source of cephalothin (25), a semisynthetic derivative of cephalosporin C, having antibiotic action similar to that of benzylpenicillin but insensitive to penicillinase. It was active against a number of penicillin resistant Staphylococcus and some Gram-negative species of bacteria. A number of chemically-related antibiotic substances named cephalosporins P1, P2, P3, P4 and P5 had been isolated from the marine species of fungus Cephalosporium acremonium. 82 Cephalosporin P1 (C33H52O8) m.p. 147°C; [α]D + 28° [CHCl3] was a mono-basic triterpenic carboxylic acid. Methylation of the acid with CH2N2 at 0°C gave a monomethyl ester m.p. 196°C, while methylation at room temperature with CH2N2 produced a product containing nitrogen. The acid