
Mam.Drugs2014,12,255-278:doi:10.3390/md12010255 OPEN ACCESS marine drugs ISSN1660-3397 www.mdpi.com/journal/marinedrugs Review Marine-Sourced Anti-Cancer and Cancer Pain Control Agents in Clinical and Late Preclinical Development David J.Newman and Gordon M.Cragg Natural Products Branch,Developmental Therapeutics Program,Division of Cancer Treatment and Diagnosis,Frederick National Laboratory,P.O.Box B,Frederick,MD 21702,USA; E-Mail:gmcragg@verizon.net The opinions expressed in this review are those of the authors,not necessarily those of the US Government. Author to whom correspondence should be addressed;E-Mail:dn22a@nih.gov; Tel:+1-301-624-1285,Fax:+1-301-631-3026 Received:5 December 2013:in revised form:17 December 2013/Accepted:7January 2014/ Published:14 January 2014 Abstract:The marine habitat has produced a significant number of very potent marine-derived agents that have the potential to inhibit the growth of human tumor cells in vitro and.in a number of cases.in both in vivo murine models and in humans.Although many agents have entered clinical trials in cancer,to date.only Cytarabine.Yondelis (ET743).Eribulin(a synthetic derivative based on the structure of halichondrin B),and the dolastatin 10 derivative,monomethylauristatin E(MMAE or vedotin)as a warhead,have been approved for use in humans (Adcetris).In this review,we show the compounds derived from marine sources that are curently in clinical trials against cancer.We have included brief discussions of the approved agents,where they are in trials to extend their initial approved activity (a common practice once an agent is approved),and have also included an extensive discussion of the use of auristatin derivatives as warheads,plus an area that has rarely been covered,the use of marine-derived agents to ameliorate the pain from cancers in humans,and to act as an adjuvant in immunological therapies. Keywords:Antibody Drug Conjugates (ADCs):marine antitumor agents;clinical trials: approved antitumor agents
Mar. Drugs 2014, 12, 255-278; doi:10.3390/md12010255 marine drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Review Marine-Sourced Anti-Cancer and Cancer Pain Control Agents in Clinical and Late Preclinical Development † David J. Newman * and Gordon M. Cragg Natural Products Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, Frederick National Laboratory, P.O. Box B, Frederick, MD 21702, USA; E-Mail: gmcragg@verizon.net † The opinions expressed in this review are those of the authors, not necessarily those of the US Government. * Author to whom correspondence should be addressed; E-Mail: dn22a@nih.gov; Tel.: +1-301-624-1285; Fax: +1-301-631-3026. Received: 5 December 2013; in revised form: 17 December 2013 / Accepted: 7 January 2014 / Published: 14 January 2014 Abstract: The marine habitat has produced a significant number of very potent marine-derived agents that have the potential to inhibit the growth of human tumor cells in vitro and, in a number of cases, in both in vivo murine models and in humans. Although many agents have entered clinical trials in cancer, to date, only Cytarabine, Yondelis® (ET743), Eribulin (a synthetic derivative based on the structure of halichondrin B), and the dolastatin 10 derivative, monomethylauristatin E (MMAE or vedotin) as a warhead, have been approved for use in humans (Adcetris®). In this review, we show the compounds derived from marine sources that are currently in clinical trials against cancer. We have included brief discussions of the approved agents, where they are in trials to extend their initial approved activity (a common practice once an agent is approved), and have also included an extensive discussion of the use of auristatin derivatives as warheads, plus an area that has rarely been covered, the use of marine-derived agents to ameliorate the pain from cancers in humans, and to act as an adjuvant in immunological therapies. Keywords: Antibody Drug Conjugates (ADCs); marine antitumor agents; clinical trials; approved antitumor agents OPEN ACCESS

Mar.Drugs 2014.12 256 1.Introduction Rather than discuss the agents that are currently in use from marine sourced organisms,which will be covered in another review in this journal,we will discuss agents that are from marine or marine-derived sources that are either in clinical trials.or are in advanced preclinical status.Obviously we will not be covering all such agents,as some are known only by a code number without any other information being available,whilst others are in"preclinical status"according to the authors of a paper or communication.but in truth.most of these are simply reports of some in vitro activity against cell inesor have some preliminary in rodents. We will also avoid using the source organism as the method of classification as it is now becoming quite evident that the majority of compounds reported from the marine environment are in fact produced by.or in concert with,single-celled organisms ranging from protists(frequently dinoflagellates) to bacteria,including a very significant number of as yet uncultured organisms. We will mention some of the materials that have been approved for use in one or more countries that are in fact in clinical trials in others,or are now being used in conjunction with other drug moieties as these are very common occurrences with antitumor agents once they are approved.For example, although not a marine-derived agent,taxo is still in clinical trials,usually as part of a multi-drug regimen more than 20 years after it was approved for use by the US Food and Drug Administration (FDA)for treatment of refractory ovarian cancer. We have organized this review in a manner that is the reverse to what most authors would do,in that we will commence with agents that have been approved but are still in clinical trials,followed by agents in stages of clinical development (nominally Phase I to IID).rather than start with preclinical agents and work forwards. Since a number of the agents that are in clinical trials are very close relatives to approved materials. we have elected to group these agents after the "approved parent",so that the similarities and differences can be more easily seen,thus giving the full"chemical lineage"in certain cases below.In addition,we have elected to commence with compounds from marine sources that could be considered as"adjuvant therapies"though,with one exception,not in the immunological sense. 2.Treatment of Pain Associated with Cancer 2.1.Tetrodotoxin (Tectin Phase Ill:Figure 1.1) One of the most unusual agents at this stage is a very well known"marine toxin",the highly substituted guanidine-derivative,tetrodotoxin(1)[1-3].Although this is not a formal anti-tumor agent, it is in fact in Phase III trials as an agent(Tectin)against inadequately controlled pain related to cancer by WEX Pharmaceuticals in the USA,together with a Phase II trial under the same company, again in the USA,against the neuropathic pain resulting from chemotherapy-induced peripheral neuropathy.Although there was debate in years gone by over the actual source of this agent,there is now little doubt that it is produced by a commensal microbe,though which one(s)is still open for debate [4].The synthesis of the compound and other derivatives has been published from a variety of chemists with an excellent recent review by Nishikawa and Isobe giving the highlights of their methodologies and covering some of the early history of this class of toxins[5]
Mar. Drugs 2014, 12 256 1. Introduction Rather than discuss the agents that are currently in use from marine sourced organisms, which will be covered in another review in this journal, we will discuss agents that are from marine or marine-derived sources that are either in clinical trials, or are in advanced preclinical status. Obviously we will not be covering all such agents, as some are known only by a code number without any other information being available, whilst others are in “preclinical status” according to the authors of a paper or communication, but in truth, most of these are simply reports of some in vitro activity against cell lines or have some preliminary data on in vivo activity in rodents. We will also avoid using the source organism as the method of classification as it is now becoming quite evident that the majority of compounds reported from the marine environment are in fact produced by, or in concert with, single-celled organisms ranging from protists (frequently dinoflagellates) to bacteria, including a very significant number of as yet uncultured organisms. We will mention some of the materials that have been approved for use in one or more countries that are in fact in clinical trials in others, or are now being used in conjunction with other drug moieties as these are very common occurrences with antitumor agents once they are approved. For example, although not a marine-derived agent, taxol® is still in clinical trials, usually as part of a multi-drug regimen more than 20 years after it was approved for use by the US Food and Drug Administration (FDA) for treatment of refractory ovarian cancer. We have organized this review in a manner that is the reverse to what most authors would do, in that we will commence with agents that have been approved but are still in clinical trials, followed by agents in stages of clinical development (nominally Phase I to III), rather than start with preclinical agents and work forwards. Since a number of the agents that are in clinical trials are very close relatives to approved materials, we have elected to group these agents after the “approved parent”, so that the similarities and differences can be more easily seen, thus giving the full “chemical lineage” in certain cases below. In addition, we have elected to commence with compounds from marine sources that could be considered as “adjuvant therapies” though, with one exception, not in the immunological sense. 2. Treatment of Pain Associated with Cancer 2.1. Tetrodotoxin (Tectin ®, Phase III; Figure 1, 1) One of the most unusual agents at this stage is a very well known “marine toxin”, the highly substituted guanidine-derivative, tetrodotoxin (1) [1–3]. Although this is not a formal anti-tumor agent, it is in fact in Phase III trials as an agent (Tectin®) against inadequately controlled pain related to cancer by WEX Pharmaceuticals in the USA, together with a Phase II trial under the same company, again in the USA, against the neuropathic pain resulting from chemotherapy-induced peripheral neuropathy. Although there was debate in years gone by over the actual source of this agent, there is now little doubt that it is produced by a commensal microbe, though which one(s) is still open for debate [4]. The synthesis of the compound and other derivatives has been published from a variety of chemists with an excellent recent review by Nishikawa and Isobe giving the highlights of their methodologies and covering some of the early history of this class of toxins [5]
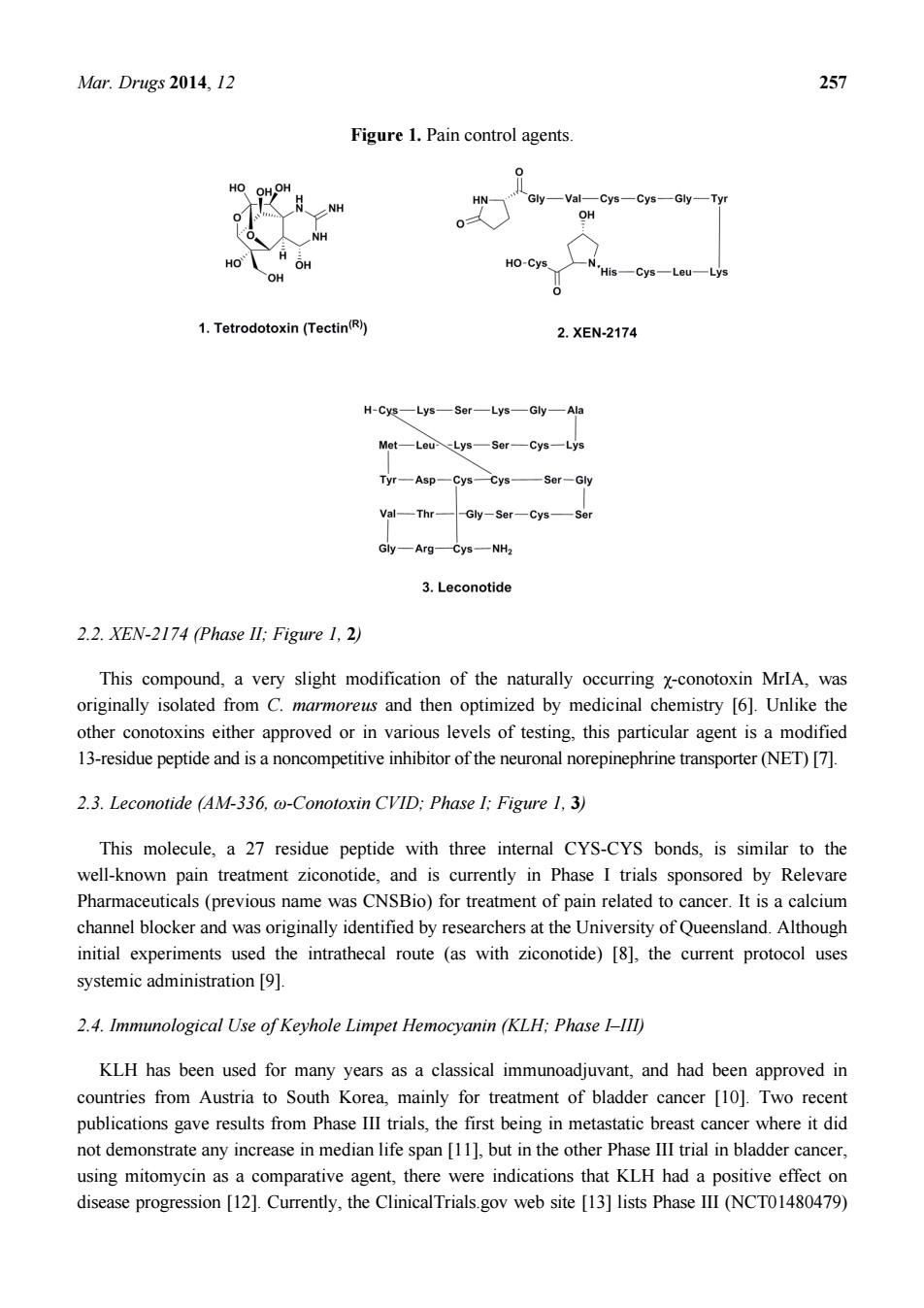
Mar.Drugs 2014,12 Figure 1.Pain control agents Gly-VatCys-Cys-Gly-Tyr OH HO-Cys 1.Tetrodotoxin(Tectin) 2.XEN-2174 H-CE一Lys—s0r一Lys一Gy一Ah Mot-Lou-Lys-Sor-Cys-Lys Tyr-Asp-Cys一cn—Ser-Gy Val一mr Gy一AgC-一NH 3.Leconotide 2.2.XEN-2174 (Phase ll:Figure 1.2) This compound,a very slight modification of the naturally occurring x-conotoxin MrlA,was originally isolated from C.marmoreus and then optimized by medicinal chemistry [6].Unlike the other conotoxins either approved or in various levels of testing,this particular agent is a modified 13-residue peptide and is a noncompetitive inhibitor of the neuronal norepinephrine transporter(NET)[7]. 2.3.Leconotide (AM-336,@-Conotoxin CVID:Phase I:Figure 1.3) This molecule,a 27 residue peptide with three internal CYS-CYS bonds,is similar to the well-known pain treatment ziconotide,and is currently in Phase I trials sponsored by Relevare Pharmaceuticals(previous name was CNSBio)for treatment of pain related to cancer.It is a calcium channel blocker and was originally identified by researchers at the University of Queensland.Although initial experiments used the intrathecal route (as with ziconotide)[8].the current protocol uses systemicadministration[9]例, 2.4.Immunological Use of Keyhole Limpet Hemocyanin (KLH:Phase I-III) KLH has been used for many years as a classical immunoadjuvant,and had been approved in countries from Austria to South Korea,mainly for treatment of bladder cancer [10].Two recent publications gave results from Phase III trials,the first being in metastatic breast cancer where it did ot demonsrate anyinreaseinedian life span[buthth Phase Iral inbladder cncer. using mitomycin as a comparative agent,there were indications that KLH had a positive effect on disease progression [12].Currently,the ClinicalTrials.gov web site [13]lists Phase IlI (NCTO1480479)
Mar. Drugs 2014, 12 257 Figure 1. Pain control agents. 2.2. XEN-2174 (Phase II; Figure 1, 2) This compound, a very slight modification of the naturally occurring χ-conotoxin MrIA, was originally isolated from C. marmoreus and then optimized by medicinal chemistry [6]. Unlike the other conotoxins either approved or in various levels of testing, this particular agent is a modified 13-residue peptide and is a noncompetitive inhibitor of the neuronal norepinephrine transporter (NET) [7]. 2.3. Leconotide (AM-336, ω-Conotoxin CVID; Phase I; Figure 1, 3) This molecule, a 27 residue peptide with three internal CYS-CYS bonds, is similar to the well-known pain treatment ziconotide, and is currently in Phase I trials sponsored by Relevare Pharmaceuticals (previous name was CNSBio) for treatment of pain related to cancer. It is a calcium channel blocker and was originally identified by researchers at the University of Queensland. Although initial experiments used the intrathecal route (as with ziconotide) [8], the current protocol uses systemic administration [9]. 2.4. Immunological Use of Keyhole Limpet Hemocyanin (KLH; Phase I–III) KLH has been used for many years as a classical immunoadjuvant, and had been approved in countries from Austria to South Korea, mainly for treatment of bladder cancer [10]. Two recent publications gave results from Phase III trials, the first being in metastatic breast cancer where it did not demonstrate any increase in median life span [11], but in the other Phase III trial in bladder cancer, using mitomycin as a comparative agent, there were indications that KLH had a positive effect on disease progression [12]. Currently, the ClinicalTrials.gov web site [13] lists Phase III (NCT01480479)

Mar.Drugs 2014,12 258 and Phase II(NCT01498328)trials using KLH in its adjuvant status against relapsed glioblastoma,and Phase I trials in conjunction with KLH as part of a vaccine against high risk neuroblastoma (NCT00911560)and fallopian tube,epithelial ovarian and peritoneal cancers in patients following a first remission(NCT01248273). 3.Approved Marine-Derived Antitumor Agents Still in Clinical Trials(and Close Chemical Relatives) 3.1.Cytarabine (Phases I to IV:Figure 2.4) As mentioned in a news interview in the early 1990s and then formally in a review by the authors in 2000 [14]this agent,though not found in a marine environment as"Ara-C"can trace its chemical lineage back to the discovery of bioactive nucleosides that contained arabinose rather than ribose or deoxyribose.Though we were not the first to recognize the importance of such substitutions,as to formally link the discoveries of the marine-sourced natural arabinoses by the Bergmann group to the design"of this agent [618].So Ara-C can be legitimately considered to be a marine-derived agent since without the arabinose.it would simply have been a normal component of nucleic acids. Even today.there are 840 trials listed in the NIH (National Institutes of Health.Bethesda MD USA)clinical trials database (ClinicalTrials.gov).with 240 of them being open studies that are recruiting,covering a large number of cancers and ranging from Phase IV down to Phase I.In the corresponding European database,43 clinical trials covering the same phases,but with some overlap are listed.As with other well-known approved drugs,it is still in use,more than 40 years after its initial approval,with an interesting recent paper questioning the use of high dose cytarabine therapy during remission in adults of acute myeloid leukemia[1] 3.2.ET743 (Trabectedin:Yondelis Phases I to Ill:Figure 2.5) This compound may well be considered the"poster child"for marine-derived antitumor agents,as it is currently the only molecule in use as an antitumor agent that is identical to one of the compounds originally isolated from E.rbinata.The stories around the discovery and development of this compound using materials from in-sea and on land aquaculture.followed by the semi-synthesis from a precursor molecule from a marine microorganism,cyanosafracin B,have been told by many authors over the years.These ranged from the initial reports of bioactivity in this organism in 1970 by Sigel et al.[20],the initial identification of the series by Holt in his PhD thesis in 1986 [21],to the simultaneous publications from the laboratories of Rinehart at the University of Illinois(Urbana Champaign,IL,USA)[22],and Wright at Harbor Branch Oceanographic Institution(Fort Pierce,FL. USA)[23]in 1990 of the structure of ET743.This work was followed with the thorough discussion given by the investigatorsat PharmaMar (Madrid,Spain)of both semi-synthesis and optimization of processes to obtain active drug principles.The molecule was approved in the EU (European Union)in 2007 for treatment of advanced soft tissue sarcoma and in some of the EU countries for treatment of recurent platinum-sensitive ovarian cance when coupled to
Mar. Drugs 2014, 12 258 and Phase II (NCT01498328) trials using KLH in its adjuvant status against relapsed glioblastoma, and Phase I trials in conjunction with KLH as part of a vaccine against high risk neuroblastoma (NCT00911560) and fallopian tube, epithelial ovarian and peritoneal cancers in patients following a first remission (NCT01248273). 3. Approved Marine-Derived Antitumor Agents Still in Clinical Trials (and Close Chemical Relatives) 3.1. Cytarabine (Phases I to IV; Figure 2, 4) As mentioned in a news interview in the early 1990s and then formally in a review by the authors in 2000 [14] this agent, though not found in a marine environment as “Ara-C” can trace its chemical lineage back to the discovery of bioactive nucleosides that contained arabinose rather than ribose or deoxyribose. Though we were not the first to recognize the importance of such substitutions, as Suckling [15] in a review in 1991 reported on the chemistry involved in the syntheses of these and other such arabinose-linked nucleosides with common or uncommon bases, we were perhaps the first to formally link the discoveries of the marine-sourced natural arabinoses by the Bergmann group to the “design” of this agent [16–18]. So Ara-C can be legitimately considered to be a marine-derived agent, since without the arabinose, it would simply have been a normal component of nucleic acids. Even today, there are 840 trials listed in the NIH (National Institutes of Health, Bethesda, MD, USA) clinical trials database (ClinicalTrials.gov), with 240 of them being open studies that are recruiting, covering a large number of cancers and ranging from Phase IV down to Phase I. In the corresponding European database, 43 clinical trials covering the same phases, but with some overlap, are listed. As with other well-known approved drugs, it is still in use, more than 40 years after its initial approval, with an interesting recent paper questioning the use of high dose cytarabine therapy during remission in adults of acute myeloid leukemia [19]. 3.2. ET743 (Trabectedin; Yondelis®; Phases I to III; Figure 2, 5) This compound may well be considered the “poster child” for marine-derived antitumor agents, as it is currently the only molecule in use as an antitumor agent that is identical to one of the compounds originally isolated from E. turbinata. The stories around the discovery and development of this compound using materials from in-sea and on land aquaculture, followed by the semi-synthesis from a precursor molecule from a marine microorganism, cyanosafracin B, have been told by many authors over the years. These ranged from the initial reports of bioactivity in this organism in 1970 by Sigel et al. [20], the initial identification of the series by Holt in his PhD thesis in 1986 [21], to the simultaneous publications from the laboratories of Rinehart at the University of Illinois (Urbana Champaign, IL, USA) [22], and Wright at Harbor Branch Oceanographic Institution (Fort Pierce, FL, USA) [23] in 1990 of the structure of ET743. This work was followed with the thorough discussion given by the investigators at PharmaMar (Madrid, Spain) in 2009 [24], demonstrating the power of both semi-synthesis and optimization of processes to obtain active drug principles. The molecule was approved in the EU (European Union) in 2007 for treatment of advanced soft tissue sarcoma and in some of the EU countries for treatment of recurrent platinum-sensitive ovarian cancer when coupled to

Mar.Drugs 2014,12 259 liposomal doxorubicin in 2009,but the corresponding U.S.FDA (Food and Drug Administration) application was withdrawn. Figure 2.Approved marine-derived drugs and close analogues in clinical trials 4.Cytarabine(Cytosar(R) 6.PM-10450(ZalypsisR) rabectedin (Yondelis 8.Eribulin (Halaven(R) 7.PM-01183(Lurbinectedin) ga,P 9.Brentuximab vedotin(AdcetrisR) 10.Monomethylauristatin E(Vedotin 11.Monomethylauristatin F
Mar. Drugs 2014, 12 259 liposomal doxorubicin in 2009, but the corresponding U.S. FDA (Food and Drug Administration) application was withdrawn. Figure 2. Approved marine-derived drugs and close analogues in clinical trials