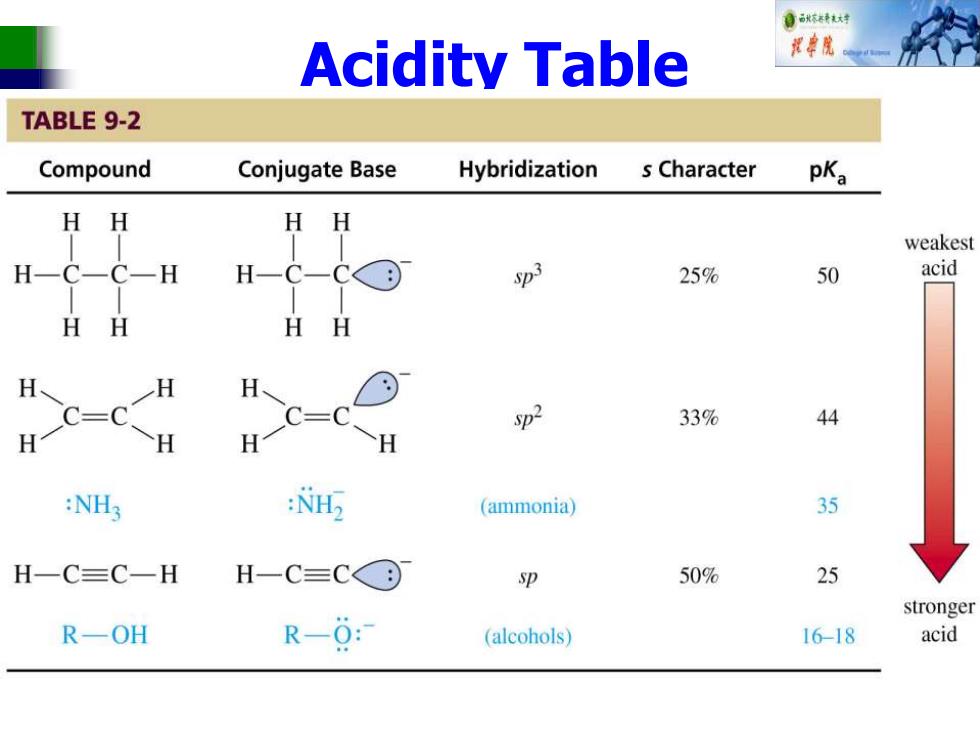
自秋不特大对 Acidity Table TABLE 9-2 Compound Conjugate Base Hybridization s Character pKa HH weakest H-C-C-H H-C-CG⊙ S3 25% 50 acid HH HH H H H C=C 一H HC-c H sp2 33% 44 H :NH3 : (ammonia) 35 H-C≡C一H H-C=C© sp 50% 25 stronger R-OH R-0: (alcohols) 16-18 acid
Acidity Table
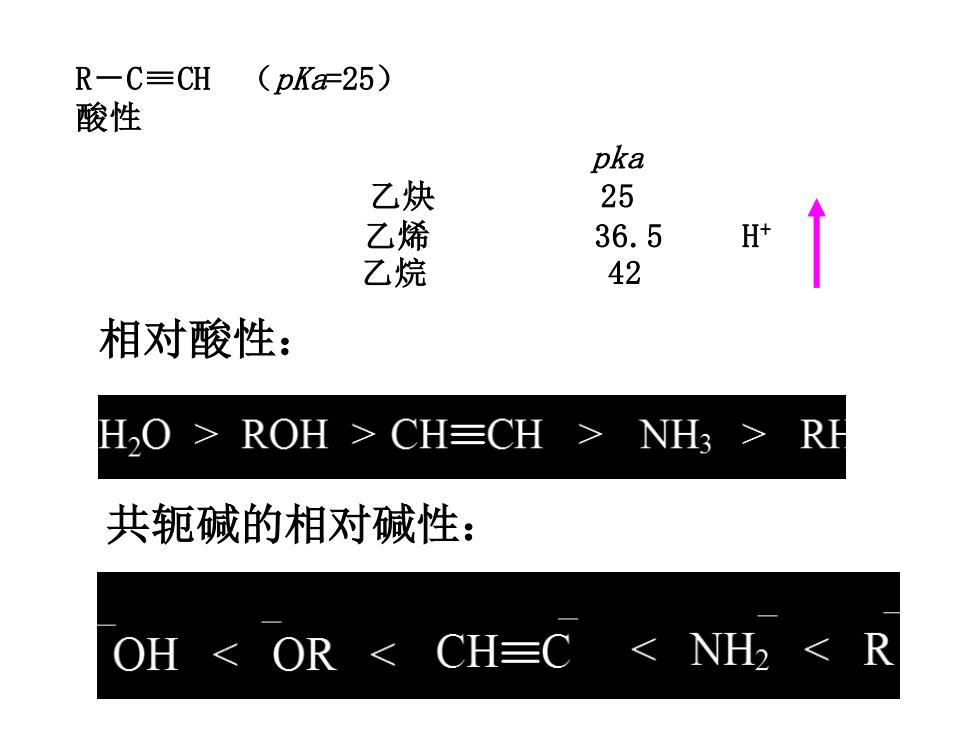
R一C=CH (pKaF25) 酸性 pka 乙炔 25 乙 36.5 H 乙烷 42 相对酸性: H2O>ROH>CH≡CH> NH3 RH 共轭碱的相对碱性: OH OR<CH≡C<NH2<R
R-C≡CH (pKa=25) 酸性 pka 乙炔 25 乙烯 36.5 H+ 乙烷 42 相对酸性: 共轭碱的相对碱性:
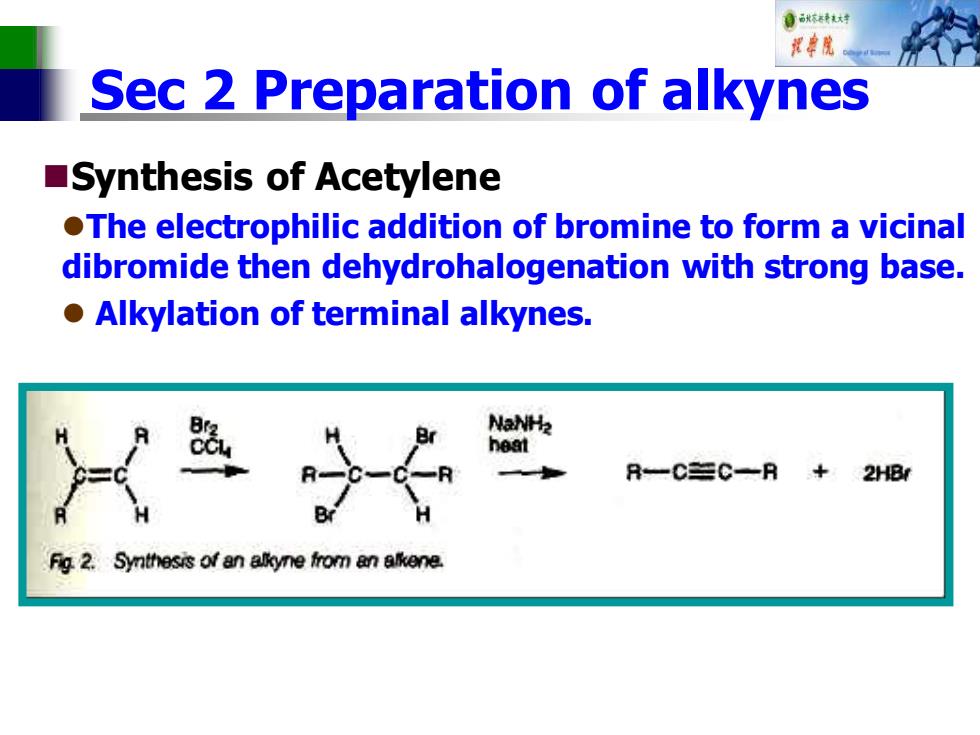
自秋不特大对 花中院。 Sec 2 Preparation of alkynes Synthesis of Acetylene OThe electrophilic addition of bromine to form a vicinal dibromide then dehydrohalogenation with strong base. o Alkylation of terminal alkynes. NaNH2 heat R一C=C一R +2H8 Fig 2.Synthesis of an allkyne from an alkene
Sec 2 Preparation of alkynes ◼Synthesis of Acetylene ⚫The electrophilic addition of bromine to form a vicinal dibromide then dehydrohalogenation with strong base. ⚫ Alkylation of terminal alkynes

自秋转达对 A Synthesis of Acetylene Heat coke with lime in an electric furnace to form calcium carbide. Then drip water on the calcium carbide. 3 C CaO CaC2 CO coke lime *CaC2+2H20 H-C≡C-H+Ca(OHD2 *This reaction was used to produce light for miners'lamps and for the stage
A Synthesis of Acetylene ◼Heat coke with lime in an electric furnace to form calcium carbide. ◼Then drip water on the calcium carbide. CaC2 + 2 H2O H C C H + Ca(OH) 2 3 C + CaO CaC2 + CO coke lime *This reaction was used to produce light for miners’ lamps and for the stage. *

B.Elimination of dihalides Removal of two molecules of HX from a vicinal or geminal dihalide produces an alkyne. First step (-HX)is easy,forms vinyl halide. Second step,removal of HX from the vinyl halide requires very strong base and high temperatures. H base base -HX -HX R R一C≡C一R (fast) (slow) R a vicinal dihalide vinyl halide alkyne H X base base -HX -HX R一C=C一RM (fast) (slow) H a geminal dihalide vinyl halide alkyne
B. Elimination of dihalides ◼Removal of two molecules of HX from a vicinal or geminal dihalide produces an alkyne. ◼First step (-HX) is easy, forms vinyl halide. ◼Second step, removal of HX from the vinyl halide requires very strong base and high temperatures