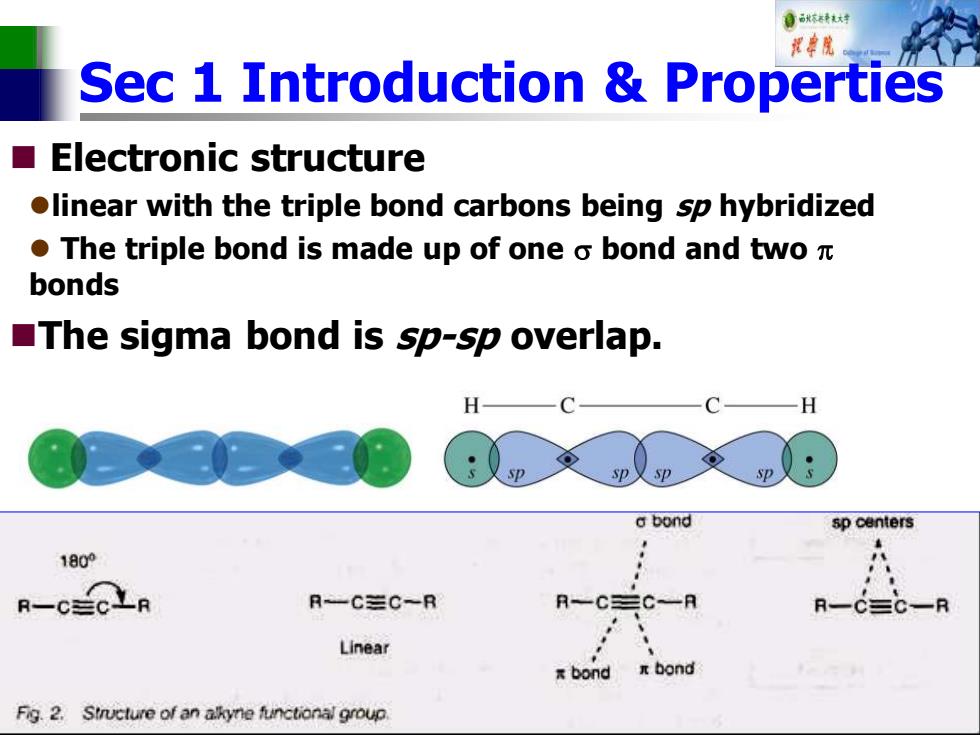
自秋转达对 视中院 Sec 1 Introduction Properties Electronic structure olinear with the triple bond carbons being sp hybridized ●The triple bond is made up of one o bond and two元 bonds The sigma bond is sp-sp overlap. H a bond sp centers 1800 R-CECLR R-C三C~R R-CEC-R Linear xbond Fig.2.Structure of an akyne functional group
Sec 1 Introduction & Properties ◼ Electronic structure ⚫linear with the triple bond carbons being sp hybridized ⚫ The triple bond is made up of one bond and two bonds ◼The sigma bond is sp-sp overlap
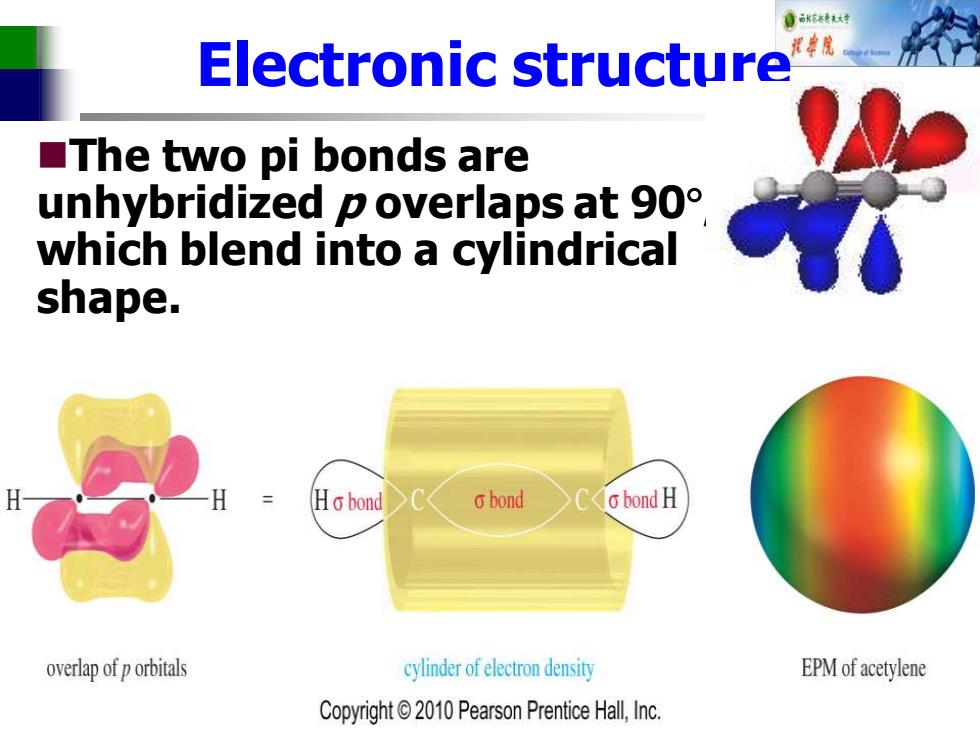
自标特花对 Electronic structure ■The two pi bonds are unhybridized p overlaps at 90 which blend into a cylindrical shape. Ho bond 6bond o bond H overlap of p orbitals cylinder of electron density EPM of acetylene Copyright2010 Pearson Prentice Hall,Inc
Electronic structure ◼The two pi bonds are unhybridized p overlaps at 90 , which blend into a cylindrical shape

自秋不转大对 Bond Lengths More s character,so shorter length. Three bonding overlaps,so shorter. 1.54A 1.33 1.20A H H H H一CC一H H H H H 1.09A 1.08A 1.06A ethane ethene ethyne Bond angle is180°,so linear geometry
Bond Lengths ◼More s character, so shorter length. ◼Three bonding overlaps, so shorter. Bond angle is 180, so linear geometry
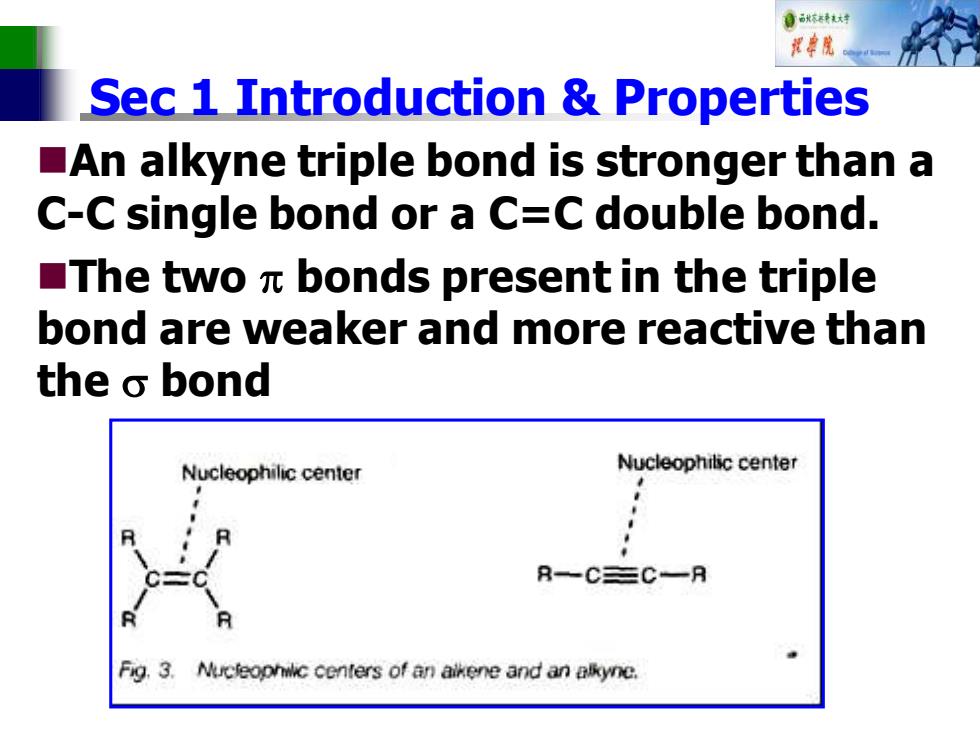
自秋特大中 花率院 Sec 1 Introduction Properties An alkyne triple bond is stronger than a C-C single bond or a C=C double bond. ■The twoπbonds present in the triple bond are weaker and more reactive than the o bond Nucleophilic center Nucleophilic center R一C三C一月 Fig.3. Nucteophilic centers of an aikene and an alkyne
Sec 1 Introduction & Properties ◼An alkyne triple bond is stronger than a C-C single bond or a C=C double bond. ◼The two bonds present in the triple bond are weaker and more reactive than the bond
自东精对 Acidity of Alkynes Terminal alkynes,R-C=C-H,are more acidic than other hydrocarbons. ■Acetylene-→acetylide by NH2,but not by OH-or RO-. More s character,so pair of electrons in anion is held more closely to the nucleus. Less charge separation,so more stable
Acidity of Alkynes ◼Terminal alkynes, R-CC-H, are more acidic than other hydrocarbons. ◼Acetylene → acetylide by NH2 - , but not by OH- or RO- . ◼More s character, so pair of electrons in anion is held more closely to the nucleus. Less charge separation, so more stable