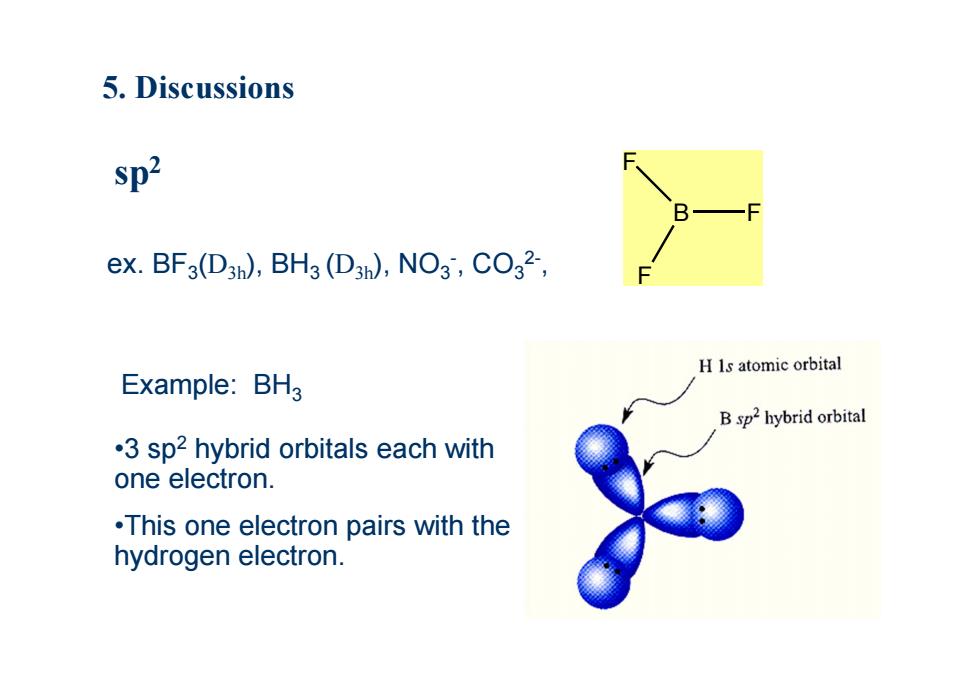
5.Discussions sp2 ex.BF3(D3h),BH3(D3n),NO3,CO32-, H Is atomic orbital Example:BH3 B sp2 hybrid orbital .3 sp2 hybrid orbitals each with one electron. .This one electron pairs with the hydrogen electron
Example: BH3 ex. BF3(D3h), BH3 (D3h), NO3-, CO32-, sp2 B F F F •3 sp2 hybrid orbitals each with one electron. •This one electron pairs with the hydrogen electron. 5. Discussions

Example: H H % H C2H4 C2H4 bond formed by t bond bond sp2-s overlap H H rmed by a bond sp2-sp2 overlap a bond (a) , (c) The sp2-hybridization and chemical bonding in C2H
tr1 tr2 x y tr3 C2H4 Example: C H H C H H C2H4 pz-pz The sp2-hybridization and chemical bonding in C2H4
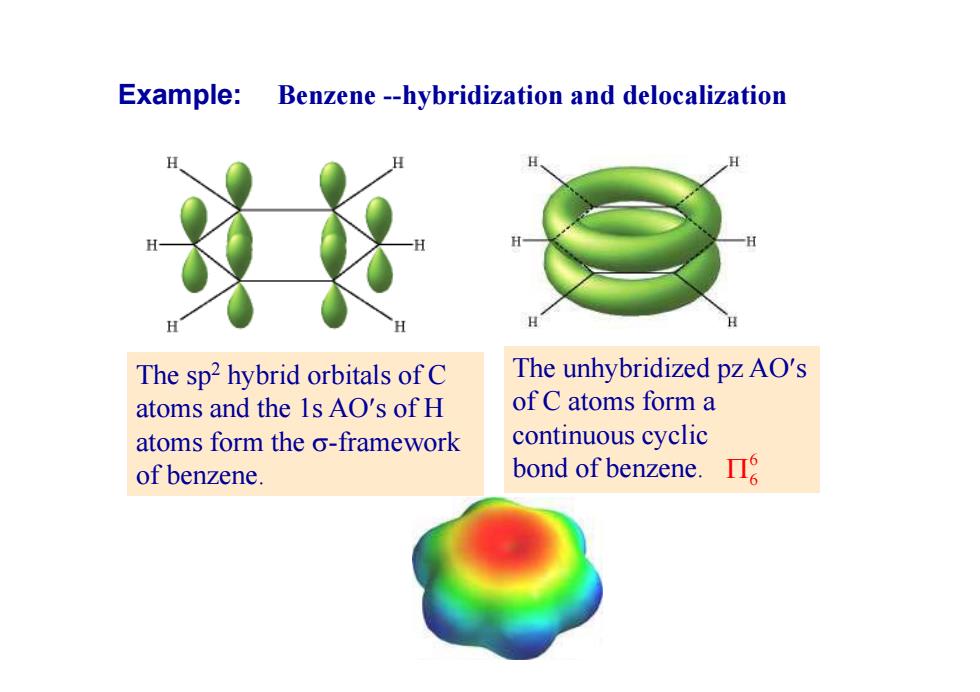
Example:Benzene--hybridization and delocalization The sp2 hybrid orbitals of C The unhybridized pz AO's atoms and the 1s AO's of H of C atoms form a atoms form the o-framework continuous cyclic of benzene bond of benzene. 哈
Example: Benzene --hybridization and delocalization The sp2 hybrid orbitals of C atoms and the 1s AOs of H atoms form the -framework of benzene. The unhybridized pz AOs of C atoms form a continuous cyclic bond of benzene. 6 6
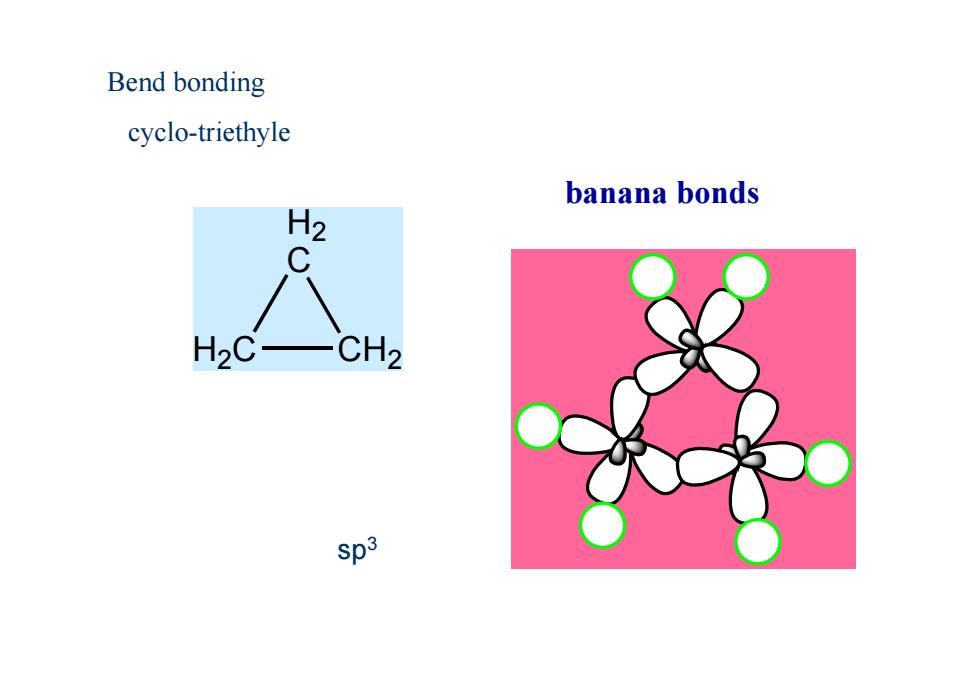
Bend bonding cyclo-triethyle banana bonds H2 H2C- CH2 Sp3
Bend bonding cyclo-triethyle H2C CH2 H2 C sp3 banana bonds

5.2 Valence Shell Electron-Pair Repulsion (VSEPR)Model 。 Quantum mechanical treatments have a number of advantages.However,the VSEPR model allows a simple qualitative prediction of molecular geometry
§5.2 Valence Shell Electron-Pair Repulsion (VSEPR) Model • Quantum mechanical treatments have a number of advantages. However, the VSEPR model allows a simple qualitative prediction of molecular geometry