
Normalization and orthogonality 0=∫0n9dr=-∫(a4.+V1-cnVa,4.+V1-a4ndr =vag∫4,'dr+VI-a)0-aj9ndr+0+0 0=Va,a,+V(1-a)1-a,)cos8, Pni cos0,=- aaj 0-4)0-ag) (c0s0,<0,0>90°) =j Equivalent hybridzation.Example:CH4 ;Non-equivalent hybridzation.Example:CHCla,CHaCl
(1 )(1 ) cos 0 (1 )(1 ) cos (1 )(1 ) 0 0 0 ( 1 )( 1 ) 2 2 2 2 i j i j ij i j i j ij i j s i j pi pj hi hj i s i pi j s j pj d d d d Equivalent hybridzation. Example: CH4 Non-equivalent hybridzation. Example: CHCl3, CH3Cl i j i j (cosij<0, ij>90°) hi hj ij Normalization and orthogonality

Equivalent hybridization sp3-hybrides 1 Q= H 4 H:C:H 1 aaj 1 H V0-a,1-4,) 3 3 $p3 4 109.5 0=109°28" 109.5° 109.5 109.5°
H C H H H sp3 hybrides 109 28" 3 1 4 3 4 1 (1 )(1 ) cos 4 1 0 i j i j ij Equivalent hybridization

Non-equivalent hybridization sp3-hybrides H:N :H H for NH3 0=107.3° a+(1-a)cos107.3°=0 0=0.23 L=1-3*0.23=0.31 Lp=3-3*0.77=0.69 Other example Wpondimg=V0.23s+V0.77p=0.48s+0.88p PHa,PF3,NF3, yoe-pmw=V0.31s+V0.69p=0.56s+0.83p
H O H H N H H sp3 hybrides X y z t1 t2 t3 t4 X y z t1 t2 t3 t4 s p s p s p s p L L for NH lone pair bonding p s 0.31 0.69 0.56 0.83 0.23 0.77 0.48 0.88 3 3*0.77 0.69 1 3*0.23 0.31 0.23 (1 ) cos107.3 0 107.3 3 Other example PH3, PF3, NF3, Non-equivalent hybridization

For d-s-p hybridization,the angles between two hybrid orbitals can be calculated by:(where a,B and y are the component of s,p and d orbitals) 3 for d'sp=1 ,B 1 6 7=3 11 13 c0s0+ 62 -cos 3 9-2-0 cos0,=0,c0s02=-1 Octahedral 01=90°,02=180°
For d-s-p hybridization, the angles between two hybrid orbitals can be calculated by: (where , and are the component of s, p and d orbitals) 90 , 180 cos 0, cos 1 ) 0 2 1 cos 2 3 ( 3 1 cos 2 1 6 1 , 3 1 , 2 1 , 6 1 for d sp , ) 0 2 1 cos 2 3 cos ( 1 2 1 2 2 2 3 2 Octahedral 5 1 3 x y z
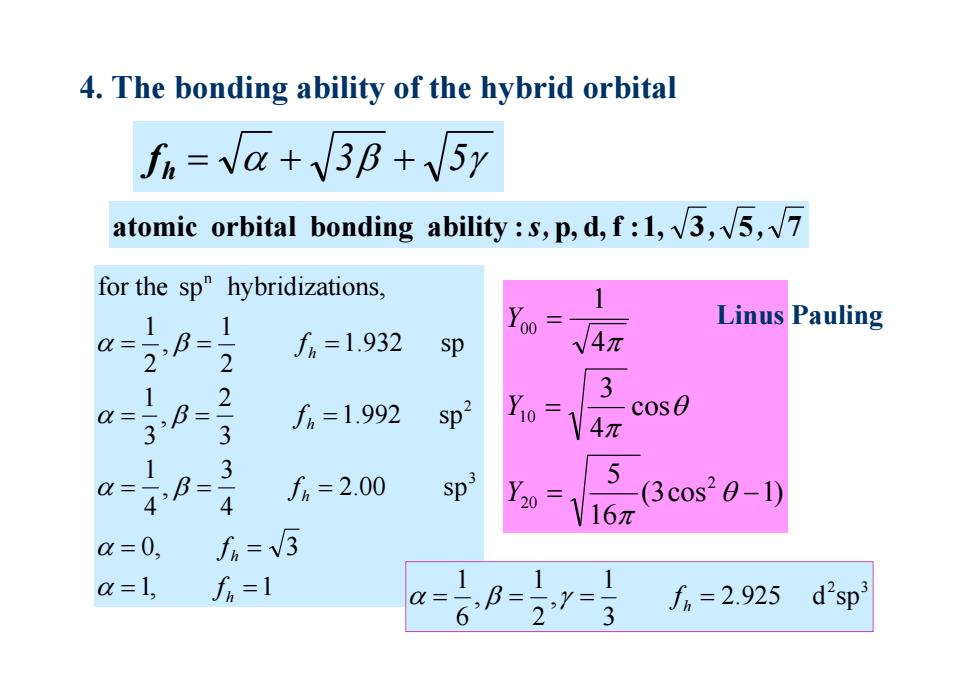
4.The bonding ability of the hybrid orbital fn =va+3B+5y atomic orbital bonding ability:s,p,d,f:l,v3,√5,√7 for the sp"hybridizations, 1 Yoo Linus Pauling Q= f6=1.932 sp √4π 2 1 2 Q-3B f%=1.992 Sp2 Y0= 3 cose 3 4π 1 a=- =3 4 f=2.00 Sp3 Y0 (3cos20-1) 1V16π a=0, fi=3 a=1, f=1 1 fm=2.925 d2sp3
3 5 hf 1, 1 0, 3 2.00 sp 43 , 41 1.992 sp 32 , 31 1.932 sp 21 , 21 for the sp hybridizations, 3 2 n h h h h h f f f f f 4. The bonding ability of the hybrid orbital atomic orbital bonding ability :s,p, d,f :1, 3 , 5 , 7 2 3 2.925 d sp 31 , 21 , 61 fh (3cos 1) 165 cos 4 3 4 1 2 20 10 00 Y Y Y Linus Pauling