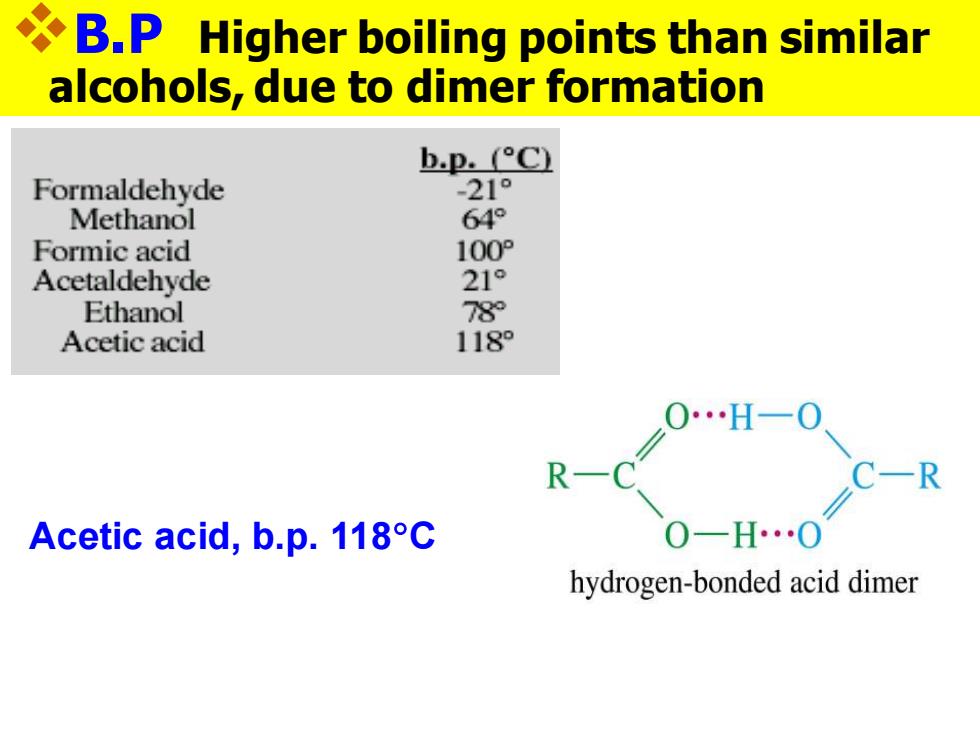
B.P Higher boiling points than similar alcohols,due to dimer formation bp.°C Formaldehyde -21° Methanol 64° Formic acid 100° Acetaldehyde 21° Ethanol 78 Acetic acid 118 0…H一0 R C-R Acetic acid,b.p.118C hydrogen-bonded acid dimer
❖B.P Higher boiling points than similar alcohols, due to dimer formation Acetic acid, b.p. 118C
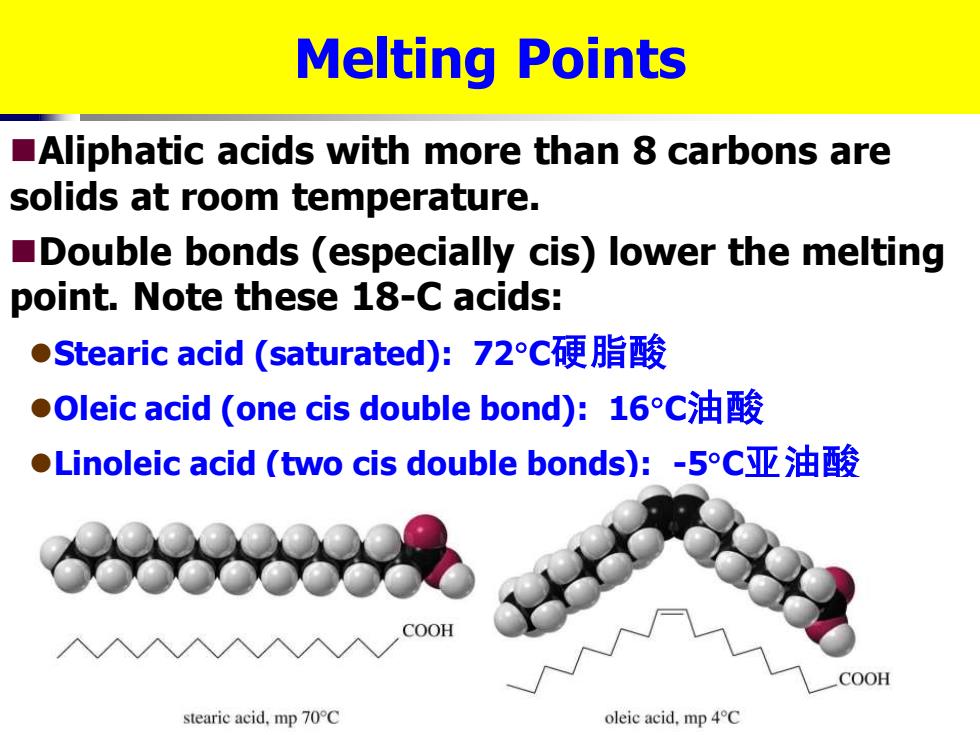
Melting Points Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis)lower the melting point.Note these 18-C acids: ●Stearic acid(saturated):72c硬脂酸 ●Oleic acid(one cis double bond):16C油酸 ●Linoleic acid(two cis double bonds):-5c亚油酸 COOH COOH stearic acid.mp70°C oleic acid.mp 4C
Melting Points ◼Aliphatic acids with more than 8 carbons are solids at room temperature. ◼Double bonds (especially cis) lower the melting point. Note these 18-C acids: ⚫Stearic acid (saturated): 72C硬脂酸 ⚫Oleic acid (one cis double bond): 16C油酸 ⚫Linoleic acid (two cis double bonds): -5C亚油酸 硬脂酸,油酸,亚油酸

Solubility Water solubility decreases with the length of the carbon chain. Up to 4 carbons,acid is miscible in water. More soluble in alcohol. Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer
Solubility ◼Water solubility decreases with the length of the carbon chain. ◼Up to 4 carbons, acid is miscible in water. ◼More soluble in alcohol. ◼Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer
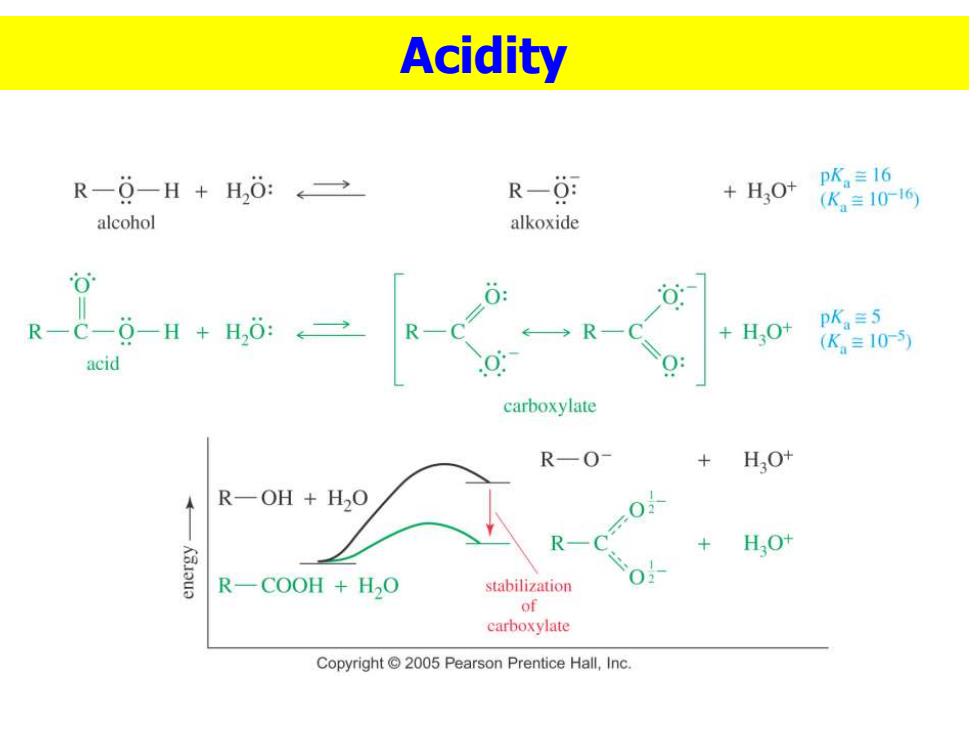
Acidity R-0-H+H,0:←→ R-0: pK。≡16 +HO+ (K。≡1016 alcohol alkoxide 0 R-C-0-H+H,:→ R HO+ pK≡5 + (K。=10-5) acid carboxylate R—O- +HO+ R-OH H2O R 0-0以 + HO+ R-COOH H2O stabilization 0 of carboxylate Copyright 2005 Pearson Prentice Hall,Inc
Acidity
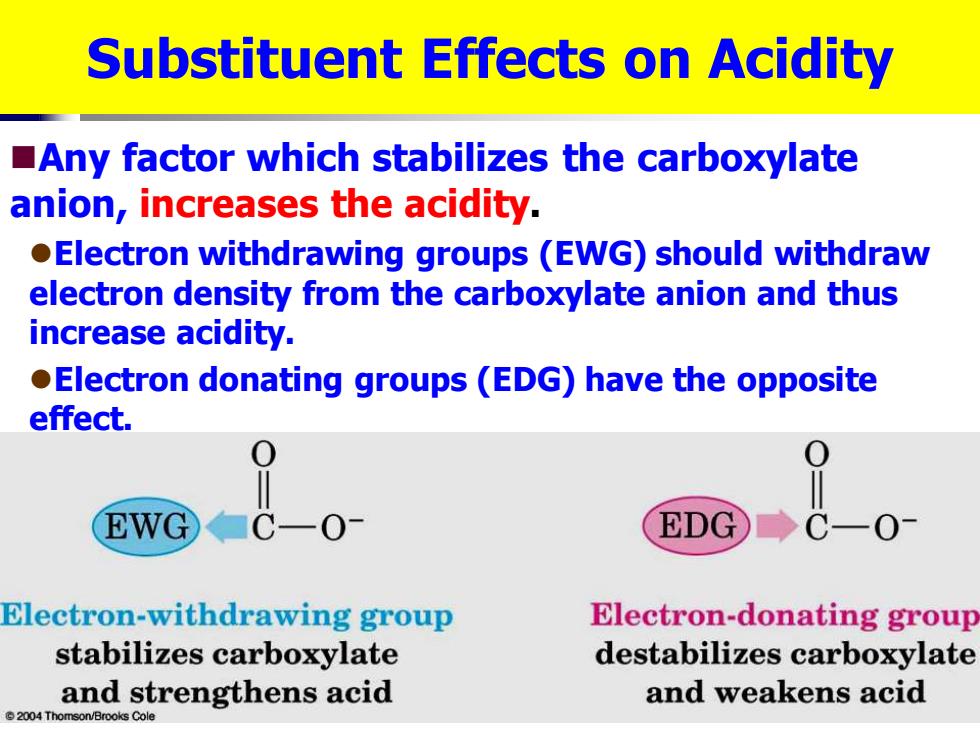
Substituent Effects on Acidity Any factor which stabilizes the carboxylate anion,increases the acidity. Electron withdrawing groups (EWG)should withdraw electron density from the carboxylate anion and thus increase acidity. Electron donating groups(EDG)have the opposite effect. EWG ■CO1 EDG C-0 Electron-withdrawing group Electron-donating group stabilizes carboxylate destabilizes carboxylate and strengthens acid and weakens acid 2004 Thomson/Brooks Cole
Substituent Effects on Acidity ◼Any factor which stabilizes the carboxylate anion, increases the acidity. ⚫Electron withdrawing groups (EWG) should withdraw electron density from the carboxylate anion and thus increase acidity. ⚫Electron donating groups (EDG) have the opposite effect