
Exercise 2 the nitrous acid solution. 3 to 4 colonies of the s usp ected streptococcal unknown are ed id s agita cells on (to suspenc rops of reagent ion)are in.som o the mixture and gently agitate again. c.One drop of the above extract is dispense with a transfer pipette onto each of two circles of the card slide.Then,one drop of the Group A latex reagent is added to one circle and one drop of the Group B latex reagent is added to the other circle. d The rocked for actants in each circle are mixed and spread out with a wooden applicator,the slide is minut nd the e results are read.A positive result will appear as a passive agglu ation reaction 6.Demonstration-observe the susceptibility of Group A streptococci to bacitracin as evidenced by the zone of inhibition around the A disc.This bacitracin sensitivity distinguishes the beta-hemolytic Group A streptococci from all the other beta-hemolvtic streptococci (Groups B.C.F,and G). 7.Demonstration-observe the susceptibility of pneumc cocci to optochin as evidenced by th zone of inhibition around the P disc.This optochin sensitivity distinguishes the pneumococci from all the other alpha-hemolytic streptococci. Sodium Bile 6.5% Bacitracin Optochin Hippurate Esculin NaCl GrouD A Strep. Beta-Hemolytic Group B Strep. Beta-Hemolytic GrouD D Entero cocc + (E.faecalis) + Strepto-coccus + moniae B.ANTIBIOTIC SUSCEPTIBILITY TEST Variations in the susceptibility of microorganisms to antibiotics are recognized within bacterial species.While some bacterial strains may naturally possess such variable susceptibilities.others may develop resistance to these therapeutic agents in vitro as well as in vivo.Infections caused by these microorganisms (e.g.,gram-negative enteric or gram-positive bacteria)thus cannot be effectively treated until information relative to their antibiotic ceptibility is obtained from the lab.Under these circumstances.and in cases where the ocess may be fatal unless treated specifically (septicemia.meningitis.etc.). selection of the most effecti tant on the ative has n isolated it is matte to dete the le antibiotics. The test is a simple one to perform banism'sd ld bace kept in mind tha n/Microb Lab Web 2001/Exercise2/exercise2.htm(4 of 10)[8/31/2002 4:30:02 PM]
the nitrous acid solution. b. With a wooden applicator, 3 to 4 colonies of the suspected streptococcal unknown are touched and emulsified into the nitrous acid solution. After some gentle agitation (to suspend the cells), 4 drops of reagent 3 (buffer solution) are added into the mixture and gently agitated again. c. One drop of the above extract is dispense with a transfer pipette onto each of two circles of the card slide. Then, one drop of the Group A latex reagent is added to one circle and one drop of the Group B latex reagent is added to the other circle. d. The reactants in each circle are mixed and spread out with a wooden applicator, the slide is rocked for 1 minute and the results are read. A positive result will appear as a passive agglutination reaction. 6. Demonstration - observe the susceptibility of Group A streptococci to bacitracin as evidenced by the zone of inhibition around the A disc. This bacitracin sensitivity distinguishes the beta-hemolytic Group A streptococci from all the other beta-hemolytic streptococci (Groups B, C, F, and G). 7. Demonstration - observe the susceptibility of pneumococci to optochin as evidenced by the zone of inhibition around the P disc. This optochin sensitivity distinguishes the pneumococci from all the other alpha-hemolytic streptococci. Bacitracin Optochin Sodium Hippurate Bile Esculin 6.5% NaCl Group A Strep. Beta-Hemolytic + - - - - Group B Strep. Beta-Hemolytic - - + - + Group D Strep. (S. bovis) - - - + - Group D Entero-cocci (E. faecalis) - - - + - Viridans Strep. Alpha-Hemolytic +- - - - - Strepto-coccus pneumoniae +- + - - - B. ANTIBIOTIC SUSCEPTIBILITY TEST Variations in the susceptibility of microorganisms to antibiotics are recognized within bacterial species. While some bacterial strains may naturally possess such variable susceptibilities, others may develop resistance to these therapeutic agents in vitro as well as in vivo. Infections caused by these microorganisms (e.g., gram-negative enteric or gram-positive bacteria) thus cannot be effectively treated until information relative to their antibiotic susceptibility is obtained from the lab. Under these circumstances, and in cases where the infectious process may be fatal unless treated specifically (septicemia, meningitis, etc.), selection of the most effective antibiotic is extremely important. Once the causative organism has been isolated, it is a routine matter to determine the organism's degree of susceptibility to available antibiotics. The test is a simple one to perform but it should be kept in mind that Exercise 2 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (4 of 10) [8/31/2002 4:30:02 PM]

Exercise 2 these in-vitro results are not always followed by clinical success.It is not uncommon that the lab's recommendation to a physician fails for various known and unknown reasons.Many variables,such as the size of the inoculum,thickness of the medium,rate of diffusion, deterioration of the antibiotic during incubation,etc.,preclude precise quantitative evaluation In spite of these limitations.however,the antibiotic susceptibility test is a necessary and useful one. ethe reliability of selecting 高的nd 0 dilution met d The Kirby-Bauer diffusion test involves using disks saturated with standard antibiotic concentrations and applying these to a large Mueller-Hinton agar plate which has been seeded with exponential phase cells of the clinical isolate.Results of this disk diffusion test are reported as the bacterium being susceptible or resistant to the various antibiotics. >>>>> Primary Specimen and Pure Culture:Kirby-Bauer Diffusion Method 1.The following exponential phase broth cultures will be available:Pseudomonas aeruginosa,Staphylococcus aureus,Escherichia coli,and Proteus mirabilis.Distribute these microorganisms so that each student at your table will use a different pure culture. 2.Insert a sterile swab into the broth using aseptic technique.Press the cotton against the side of the tube to remove some of the excess fluid. plate by streaking the swal half the plate is 4.Rotate the plate 90 and repeat the streaking with the swab(you may have to obtain some sp n with another sterile swab)Continue tati and streaking until the e covered.An evenly distributed population of microorganisms throughout the agar is desired. 5.Allow the plates to dry for 5 to 10 minutes and then apply 12 antibiotic discs with a Sensi-disc dispenser using aseptic technique.Press these discs gently with alcohol-flamed teasing needle and incubate overnight at 37C 6.Make sure you record name.code and concentration of each antibiotic. SECOND SESSION FOCL file://C/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (5 of 10)[34:30:02 PM
these in-vitro results are not always followed by clinical success. It is not uncommon that the lab's recommendation to a physician fails for various known and unknown reasons. Many variables, such as the size of the inoculum, thickness of the medium, rate of diffusion, deterioration of the antibiotic during incubation, etc., preclude precise quantitative evaluation. In spite of these limitations, however, the antibiotic susceptibility test is a necessary and useful one. Many different systems have been proposed in attempts to improve the reliability of selecting the best antibiotic for the patient on the basis of test results. Currently, only two systems are primarily used in the clinical lab and these are the MIC dilution method and the Kirby-Bauer diffusion method. The Kirby-Bauer diffusion test involves using disks saturated with standard antibiotic concentrations and applying these to a large Mueller-Hinton agar plate which has been seeded with exponential phase cells of the clinical isolate. Results of this disk diffusion test are reported as the bacterium being susceptible or resistant to the various antibiotics. >>>>> Primary Specimen and Pure Culture: Kirby-Bauer Diffusion Method 1. The following exponential phase broth cultures will be available: Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, and Proteus mirabilis. Distribute these microorganisms so that each student at your table will use a different pure culture. 2. Insert a sterile swab into the broth using aseptic technique. Press the cotton against the side of the tube to remove some of the excess fluid. 3. Prepare a confluent lawn of bacteria on a Mueller-Hinton agar plate by streaking the swab from edge to edge, moving from the periphery to the center of plate until half the plate is covered. 4. Rotate the plate 90° and repeat the streaking with the swab (you may have to obtain some more specimen with another sterile swab). Continue rotating and streaking until the entire plate is covered. An evenly distributed population of microorganisms throughout the agar is desired. 5. Allow the plates to dry for 5 to 10 minutes and then apply 12 antibiotic discs with a Sensi-disc dispenser using aseptic technique. Press these discs gently with alcohol-flamed teasing needle and incubate overnight at 37°C. 6. Make sure you record name, code and concentration of each antibiotic. SECOND SESSION: FOCI: Exercise 2 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (5 of 10) [8/31/2002 4:30:02 PM]

Exercise 2 1.Identification of Staphylococcus by Macroscopic,Microscopic and Metabolic Characteristics. 2. Antibiotic susceptibility test,reading of results A.STAPHYLOCOCCUS Staphylococci are t ubiquitous orga sms associated with man and are two spe sually encountered are Sr ofoccus direus (pathogen)and S (opportu norma ora microorganism) icroscopically, Staphylococcus aureus disease in man includes wound infections,boils,carbuncles,impetigo. meningitis.endocarditis.osteomyelitis and food poisoning.This microorganism also plays an important role in hospital-acquired infections.Various strains of S.epidermidis,S. haemolyricus,and other species(referred to as coagulase-negative staphylococci)may also be implicated in causing infections especially in the presence of foreign bodies such as implants and catheters.In the laboratory,the production of hemolysin,fermentation of mannitol,and a Dositive coagulase test have b ed as indicators of pathogenicity (for S.aureus)but had the highest degree Staphylococcal diseases are haracterized by suppuration although the mechanisms involved in pathogenicity are not well defir ed.Such factors may include antiphagocytic surface components(protein A),increased resistance to intracellular killing.production of alpha-toxin (hemolysin)which may promote necrosis,leucocidins,staphylokinase and hyaluronidase.S. aureus may also produce an enterotoxin which is the cause of staphylococcal food poisoning Staphylococci are notable for developing resistance to antimicrobial drugs including the penicillins.The determination of penicillinase(beta-lactamase)production and resistance to macrolide antibiotics are transferable by transduction and are believed to be controlled by a plasmid. 1.Each student will receive a TSAculture of either reusor Staphylococcu epidermidis.The following should be performed for identification and characterization of each microorganism. 2.Describe the cultural characteristics of the two staphylococcal species on blood agar medium.Note and record the type,size,and color of the colonies and describe the type of hemolysis produced by S.aureus. Staphylococcus aureus Staphylococcus epidermidis 3.Gram stain the bacteria and note the morphology and staining characteristics(the cocc average .8-1.0 micron in diameter). Staphylococcus aureus Staphylococcus epidermidis 4.Perform a catalase slide test by emulsifying some visible growth in a drop of hydrogen ci are catalase gative. 5.Coagulase Test-Perform the coagulase test for presence of coagulase by inoculating a tube containing 0.5 ml of rabbit plasma with your staphylococcal culture.Incubate this at 37oC and examine after 2-3 hours or after ovenight incubation.S.aureus will gel the plasma file://CWINDC p/clown/Microb Lab Web 2001/Exercise2/exercise .htm(6of10)[8/31/20024:30:02PM
Identification of Staphylococcus by Macroscopic, Microscopic and Metabolic Characteristics. 1. 2. Antibiotic susceptibility test, reading of results A. STAPHYLOCOCCUS Staphylococci are among the most ubiquitous organisms associated with man and are common inhabitants of the skin, oral cavity, and nasopharynx. the two species usually encountered are Staphylococcus aureus (pathogen) and Staphylococcus epidermidis (opportunist, normal skin flora microorganism). Microscopically, both species appear as gram-positive cocci in grape-like clusters and therefore are morphologically indistinguishable. Staphylococcus aureus disease in man includes wound infections, boils, carbuncles, impetigo, meningitis, endocarditis, osteomyelitis and food poisoning. This microorganism also plays an important role in hospital-acquired infections. Various strains of S. epidermidis, S. haemolyticus, and other species (referred to as coagulase-negative staphylococci) may also be implicated in causing infections especially in the presence of foreign bodies such as implants and catheters. In the laboratory, the production of hemolysin, fermentation of mannitol, and a positive coagulase test have been used as indicators of pathogenicity (for S. aureus) but coagulase production had the highest degree of correlation. Staphylococcal diseases are characterized by suppuration although the mechanisms involved in pathogenicity are not well defined. Such factors may include antiphagocytic surface components (protein A), increased resistance to intracellular killing, production of alpha-toxin (hemolysin) which may promote necrosis, leucocidins, staphylokinase and hyaluronidase. S. aureus may also produce an enterotoxin which is the cause of staphylococcal food poisoning. Staphylococci are notable for developing resistance to antimicrobial drugs including the penicillins. The determination of penicillinase (beta-lactamase) production and resistance to macrolide antibiotics are transferable by transduction and are believed to be controlled by a plasmid. >>>>> 1. Each student will receive a TSA culture of either Staphylococcus aureus or Staphylococcus epidermidis. The following should be performed for identification and characterization of each microorganism. 2. Describe the cultural characteristics of the two staphylococcal species on blood agar medium. Note and record the type, size, and color of the colonies and describe the type of hemolysis produced by S. aureus. Staphylococcus aureus Staphylococcus epidermidis 3. Gram stain the bacteria and note the morphology and staining characteristics (the cocci average 0.8-1.0 micron in diameter). Staphylococcus aureus Staphylococcus epidermidis 4. Perform a catalase slide test by emulsifying some visible growth in a drop of hydrogen peroxide. This reaction is positive if there is a rapid ebullition of gas. Micrococci and Staphylococci are catalase-positive; Streptococci and Pneumococci are catalase-negative. 5. Coagulase Test - Perform the coagulase test for presence of coagulase by inoculating a tube containing 0.5 ml of rabbit plasma with your staphylococcal culture. Incubate this at 37oC and examine after 2-3 hours or after ovenight incubation. S. aureus will gel the plasma Exercise 2 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (6 of 10) [8/31/2002 4:30:02 PM]
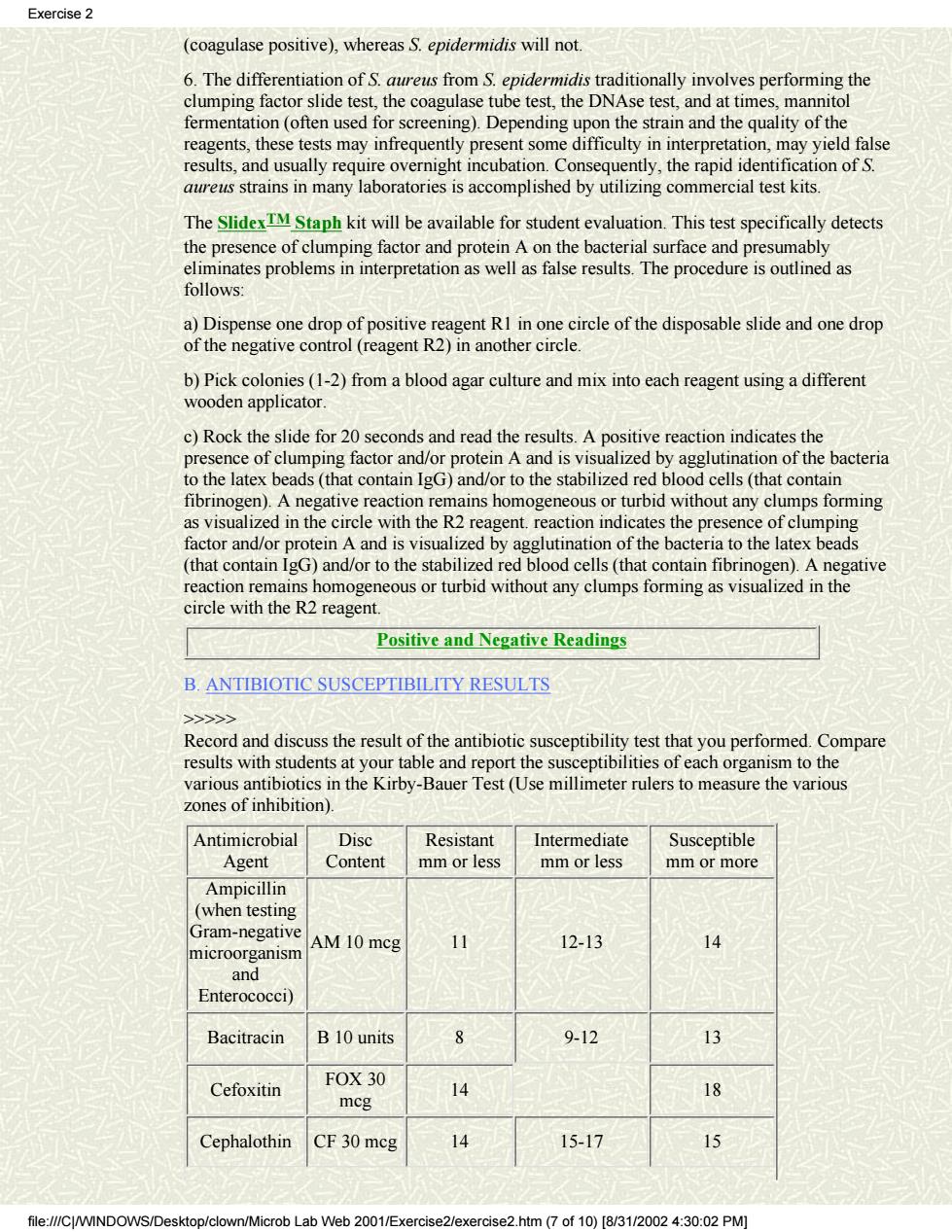
Exercise 2 (coagulase positive),whereas S.epidermidis will not. the coag time: ferment on(often used for screening).Depending upon th reagents,t ese te results,and usually require overnight incubation.Consequently,the rapid identifi on of S. aureus strains in many laboratories is accomplished by utilizing commercial test kits. The SlidexTM Staph kit will be available for student evaluation.This test specifically detects the presence of clumping facto and protein A on the bacterial surface d presu re is outlined as follows: a)Dispense one drop of positive reagent RI in one circle of the disposable slide and one drop of the negative control (reagent R2)in another circle. b)Pick colonies(1-2)from a blood agar culture and mix into each reagent using a different wooden applicator. c)Rock the slide for 20 seconds and read the results.A positiv ereaction indicates the presence of clumping factor and/or protein A and i isualized by agglutinat on of the bacteria to the latex beads(that contain IgG)and/or to the stabilized red blood cells(that contain fibrinogen).A negative reaction remains homogeneous or turbid without any clumps forming as visualized in the circle with the R2 reagent.reaction indicates the presence of clumping factor and/or protein A and is visualized by agglutination of the bacteria to the latex beads (that contain IgG)and/or to the stabilized red blood cells(that contain fibrinogen).A negative reaction remains homogeneous or turbid without any clumps forming as visualized in the circle with the R2 reagent. Positive and Negative Readings B.ANTIBIOTIC SUSCEPTIBILITY RESULTS >> Record anddiscus theesoftheantiboi suceptibiity test thatyouperfomed.Compare results with students at your table and report the susceptibilities of each organism to the various antibiotics in the Kirby-Bauer Test(Use millimeter rulers to measure the various zones of inhibition). Antimicrobial Disc Resistant Intermediate Susceptible Agent Content mm or less mm or less mm or more Ampicillin (when testing Gram-negative AM 10 mcg 11 12-13 microorganisn 14 and Enterococci) Bacitracin B10 units 9-12 Cefoxitin FOX 30 mcg 14 伊 Cephalothin CF 30 mcg 14 15-17 15 file://C/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (7of10)[8/3/002:30:02 PM)
(coagulase positive), whereas S. epidermidis will not. 6. The differentiation of S. aureus from S. epidermidis traditionally involves performing the clumping factor slide test, the coagulase tube test, the DNAse test, and at times, mannitol fermentation (often used for screening). Depending upon the strain and the quality of the reagents, these tests may infrequently present some difficulty in interpretation, may yield false results, and usually require overnight incubation. Consequently, the rapid identification of S. aureus strains in many laboratories is accomplished by utilizing commercial test kits. The SlidexTM Staph kit will be available for student evaluation. This test specifically detects the presence of clumping factor and protein A on the bacterial surface and presumably eliminates problems in interpretation as well as false results. The procedure is outlined as follows: a) Dispense one drop of positive reagent R1 in one circle of the disposable slide and one drop of the negative control (reagent R2) in another circle. b) Pick colonies (1-2) from a blood agar culture and mix into each reagent using a different wooden applicator. c) Rock the slide for 20 seconds and read the results. A positive reaction indicates the presence of clumping factor and/or protein A and is visualized by agglutination of the bacteria to the latex beads (that contain IgG) and/or to the stabilized red blood cells (that contain fibrinogen). A negative reaction remains homogeneous or turbid without any clumps forming as visualized in the circle with the R2 reagent. reaction indicates the presence of clumping factor and/or protein A and is visualized by agglutination of the bacteria to the latex beads (that contain IgG) and/or to the stabilized red blood cells (that contain fibrinogen). A negative reaction remains homogeneous or turbid without any clumps forming as visualized in the circle with the R2 reagent. Positive and Negative Readings B. ANTIBIOTIC SUSCEPTIBILITY RESULTS >>>>> Record and discuss the result of the antibiotic susceptibility test that you performed. Compare results with students at your table and report the susceptibilities of each organism to the various antibiotics in the Kirby-Bauer Test (Use millimeter rulers to measure the various zones of inhibition). Antimicrobial Agent Disc Content Resistant mm or less Intermediate mm or less Susceptible mm or more Ampicillin (when testing Gram-negative microorganism and Enterococci) AM 10 mcg 11 12-13 14 Bacitracin B 10 units 8 9-12 13 Cefoxitin FOX 30 mcg 14 18 Cephalothin CF 30 mcg 14 15-17 15 Exercise 2 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (7 of 10) [8/31/2002 4:30:02 PM]

Exercise2 Erythromycin E15 mcg 13 14-17 Gentamicin GM 10 meg 12 13-14 Oxacillin OX 1 mcg 10 11-12 Rifampin RA 5 mcg 24 25 Tetracycline Te 30 mcg 14 15-18 9 ibited b Tonamides)on the usual dosage schedule I-Intermediate-Inhibitory zone between inner and outer zone circles. S-Sensitive-Inhibitory zone equal to or greater than outer zone circle.The organism may be expected to be inhibited by concentrations of the drug attainable in the blood (or urine in the case of Nalidixic Acid Nitrofurantoin and sulfonan nides)on the isual dosage schedule. Outer one circle Innerzone circle If the organisms used for the Kirb y-Bauer were actual isol lates causing septicemia or a postoperative abscess,which antibiotic would you prescribe for treatment? file:///C/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm(8 of 10)[8/31/2002 4:30:02 PM]
Erythromycin E 15 mcg 13 14-17 18 Gentamicin GM 10 mcg 12 13-14 15 Oxacillin OX 1 mcg 10 11-12 13 Rifampin RA 5 mcg 24 25 Tetracycline Te 30 mcg 14 15-18 19 R-Resistant-Inhibitory zone equal to or less than inner zone circle. The organism is not inhibited by concentrations of the drug attainable in the blood (or urine in the case of Naladixic Acid, Nitrofurantoin and sulfonamides) on the usual dosage schedule. I-Intermediate-Inhibitory zone between inner and outer zone circles. S-Sensitive-Inhibitory zone equal to or greater than outer zone circle. The organism may be expected to be inhibited by concentrations of the drug attainable in the blood (or urine in the case of Nalidixic Acid Nitrofurantoin and sulfonamides) on the usual dosage schedule. If the organisms used for the Kirby-Bauer were actual isolates causing septicemia or a postoperative abscess, which antibiotic would you prescribe for treatment? Exercise 2 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (8 of 10) [8/31/2002 4:30:02 PM]