
Medical Microbiology Laboratory Cover A Digital Manual for Medical Exercisel Exercise2 Microbiology Laboratory. Exercise3 Exercise4 By Dr.Marion Chan,Ph.D. Exercise5 The manual is constructed as a collaborative effort of the Department of Microbiology and Immunology,School of Medicine and School of Podiatric Exercise6 Medicine,Temple University.Colored images of cultures and reactions are provided to allow students to prepare for the exercises as well as review them for Exercise7 exams Clinical Correspondence: case studies Suggestions Dr.Marion Chan,Ph.D.,Assistant Professor,Department of Microbiology and Immunology,Temple University School of Medicine,3400 North Broad Hit Street,Philadelphia,PA,19140. Te:215-707-82620r215-625-5270 marionc@astro.temple.edu Acknowledgement: The authors wish to thank Mr.Ernesto Mu ujorra and Mr.Victor Thompson for their excellent assistance in obtaining some of the microscopic and macroscopic images Drs.Robert Christman and David Grainger for assistance in computer technology Dr.R.Tuan of Thomas Jefferson University and Division of Life Science of Rutgers University have generously allowed us to use their equipment for obtaining the microscopic images. Copyright of Temple University.All rights reserved. Last modified:Sunday September 09,2001. file://CJ/WINDOWS/D sktop/clown/Microb Lab Web 2001/medicalmic ologylaboratory2001.htm [8/31/2002 4:29:46 PM)
Cover Exercise1 Exercise2 Exercise3 Exercise4 Exercise5 Exercise6 Exercise7 Clinical case studies Suggestions Hit Counter A Digital Manual for Medical Microbiology Laboratory. By Dr. Marion Chan, Ph.D. The manual is constructed as a collaborative effort of the Department of Microbiology and Immunology, School of Medicine and School of Podiatric Medicine, Temple University. Colored images of cultures and reactions are provided to allow students to prepare for the exercises as well as review them for exams. Correspondence: Dr. Marion Chan, Ph.D., Assistant Professor, Department of Microbiology and Immunology, Temple University School of Medicine, 3400 North Broad Street, Philadelphia, PA, 19140. Tel: 215-707-8262 or 215-625-5270 marionc@astro.temple.edu Acknowledgement: The authors wish to thank Mr. Ernesto Mujorra and Mr. Victor Thompson for their excellent assistance in obtaining some of the microscopic and macroscopic images. Drs. Robert Christman and David Grainger for assistance in computer technology Dr. R. Tuan of Thomas Jefferson University and Division of Life Science of Rutgers University have generously allowed us to use their equipment for obtaining the microscopic images. Copyright of Temple University. All rights reserved. Last modified: Sunday September 09, 2001. Medical Microbiology Laboratory file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/medicalmicrobiologylaboratory2001.htm [8/31/2002 4:29:46 PM]

Exercise 1 Cover EXERCISE #1 MICROSCOPY,BACTERIAL ISOLATION AND STAINING. Exercisel FIRST SESSION Exercise2 FOCI:Aseptic Technique,Staining,Microscopic Observation of Bacteria Exercise3 and Bacterial Isolation Using Streak Plate Method. Exercise4 A.Aseptic Technique and Isolation of Bacteria colonies Exercise5 Man harbors a wide variety of microorganisms on the skin and in the oral cavity,nasopharynx and intestinal tract.In all these areas of the body,many Exercise6 different kinds of bacteria exist together.Thus,if they are to be studied individually,each species must be isolated and then sub-cultured to obtain a Exercise7 pure culture.This is particularly true in the study of disease where it is Clinical necessary to isolate and identify the specific etiologic agent.Each of the case studies students is to develop lab skills that are collectively referred to as aseptic technique.This concept must be consistently kept in mind when handling Suggestions bacterial cultures because the careless student will contaminate everything including the pure cultures of bacteria provided for the study of general Hit Counter bacteriology.Without pure cultures this study becomes an effort in futility Aseptic technique will be demonstrated by the lab instructor and the studen should become quite familiar with the procedure to aseptically transfer bacteria from pure cultures. In general,pure cultures of isolated bacterial colonies are best obtained by using solid media and streak plates are primarily used for this purpose. y Each student will receive a tryptic soy agar plate and a tube of broth containing a mixture of bac eria.The "fou -way "method will be used to streak the plate.This will be demonstrated by the instructor and is described below. 1.Never remove the top of the plate but lift the lid just high enough to make complete strokes 2.Place a loop of organisms on one edge of the agar(area 1)and then streak back and forth,overlapping the streaks until you have covered about one fourth of the plate. 3.Flame the loop before proceeding to each of the other steps 4.Turn the plate approximately 90 and start streaking from one corner of the streaked area 1 and cover about one fourth of the surface(area 2).Turn again and streak surface area 3 by overlapping area 2,as shown below. Repeat this method and streak the surface of area 4 but do not run the final streaks back into any of the previous areas.You have now spread the file://C/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm(1 of6)[8/3/00 9:54 PM
Cover Exercise1 Exercise2 Exercise3 Exercise4 Exercise5 Exercise6 Exercise7 Clinical case studies Suggestions Hit Counter EXERCISE #1 MICROSCOPY, BACTERIAL ISOLATION AND STAINING. FIRST SESSION FOCI: Aseptic Technique, Staining, Microscopic Observation of Bacteria, and Bacterial Isolation Using Streak Plate Method. A. Aseptic Technique and Isolation of Bacteria colonies Man harbors a wide variety of microorganisms on the skin and in the oral cavity, nasopharynx and intestinal tract. In all these areas of the body, many different kinds of bacteria exist together. Thus, if they are to be studied individually, each species must be isolated and then sub-cultured to obtain a pure culture. This is particularly true in the study of disease where it is necessary to isolate and identify the specific etiologic agent. Each of the students is to develop lab skills that are collectively referred to as aseptic technique. This concept must be consistently kept in mind when handling bacterial cultures because the careless student will contaminate everything, including the pure cultures of bacteria provided for the study of general bacteriology. Without pure cultures this study becomes an effort in futility! Aseptic technique will be demonstrated by the lab instructor and the student should become quite familiar with the procedure to aseptically transfer bacteria from pure cultures. In general, pure cultures of isolated bacterial colonies are best obtained by using solid media and streak plates are primarily used for this purpose. >>>>> Each student will receive a tryptic soy agar plate and a tube of broth containing a mixture of bacteria. The "four-way" method will be used to streak the plate. This will be demonstrated by the instructor and is described below. 1. Never remove the top of the plate but lift the lid just high enough to make complete strokes. 2. Place a loop of organisms on one edge of the agar (area 1) and then streak back and forth, overlapping the streaks until you have covered about one fourth of the plate. 3. Flame the loop before proceeding to each of the other steps 4. Turn the plate approximately 90° and start streaking from one corner of the streaked area 1 and cover about one fourth of the surface (area 2). Turn again and streak surface area 3 by overlapping area 2, as shown below. Repeat this method and streak the surface of area 4 but do not run the final streaks back into any of the previous areas. You have now spread the Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (1 of 6) [8/31/2002 4:29:54 PM]
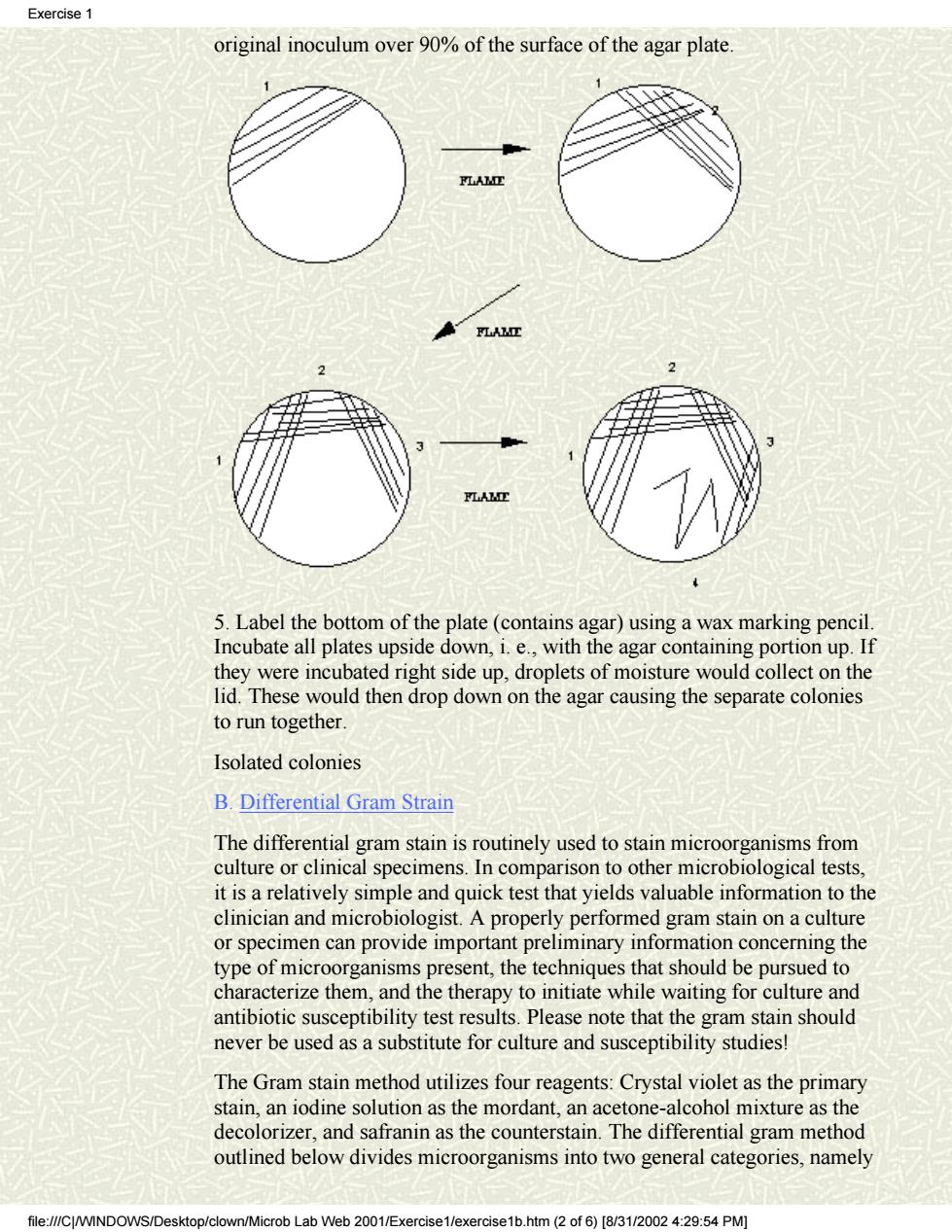
Exercise original inoculum over 90%of the surface of the agar plate 5.Label the bottom of the plate(contains agar)using a wax marking pencil Incubate all plates upside down,i.e.,with the agar containing portion up.If they were incubated right side up,droplets of moisture would collect on the lid.These would then drop down on the agar causing the separate colonies to run together Isolated colonies B.Differential Gram Strain The differential gram stain is routinely used to stain microorganisms from culture or clinical specimens.In comparison to other microbiological tests. it is a relatively simple and quick test that yields valuable information to the clinician and microbiologist.A properly performed gram stain on a culture or specimen can provide important preliminary information concerning the type of microorganisms present,the techniques that should be pursued to characterize them,and the therapy to initiate while waiting for culture and antibiotic susceptibility test results.Please note that the gram stain should never be used as a substitute for culture and susceptibility studies! The Gram stain method utilizes four reagents:Crystal violet as the primary stain,an iodine solution as the mordant,an acetone-alcohol mixture as the decolorizer,and safranin as the counterstain.The differential gram method outlined below divides microorganisms into two general categories,namely rob Lab Web 2001/E× htm2of6)[831/20024:29:54PM
original inoculum over 90% of the surface of the agar plate. 5. Label the bottom of the plate (contains agar) using a wax marking pencil. Incubate all plates upside down, i. e., with the agar containing portion up. If they were incubated right side up, droplets of moisture would collect on the lid. These would then drop down on the agar causing the separate colonies to run together. Isolated colonies B. Differential Gram Strain The differential gram stain is routinely used to stain microorganisms from culture or clinical specimens. In comparison to other microbiological tests, it is a relatively simple and quick test that yields valuable information to the clinician and microbiologist. A properly performed gram stain on a culture or specimen can provide important preliminary information concerning the type of microorganisms present, the techniques that should be pursued to characterize them, and the therapy to initiate while waiting for culture and antibiotic susceptibility test results. Please note that the gram stain should never be used as a substitute for culture and susceptibility studies! The Gram stain method utilizes four reagents: Crystal violet as the primary stain, an iodine solution as the mordant, an acetone-alcohol mixture as the decolorizer, and safranin as the counterstain. The differential gram method outlined below divides microorganisms into two general categories, namely Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (2 of 6) [8/31/2002 4:29:54 PM]

Exercise 1 gram-positive and gram-negative bacteria.The difference between these bacteria is that gram positives are not decolorized when alcohol or acetone is applied to the smear.They thus retain the primary stain and are colored purple when observed microscopically under oil immersion.Gram-negative bacteria,however,lose the primary stain upon decolorization and appear pink or red because of the safranin counterstain. >>>>> 1.To prepare a smear from a broth culture(liquid suspension of bacterial cells),merely spread a loopful of the culture over a 2 cm square area of the microscope slide.Aseptic technique must be used to accomplish this transfer and remember to ALWAYS FLAME LOOP BEFORE AND AFTER USE!! 2.For colonies on agar plate,place a loop(or two)of water on a glass slide Using aseptic technique,gently touch the bacterial surface growth with an inoculating needle or loop and remove a small part of the colony.Emulsify the bacteria on the loop into the drop of water until it just looks cloudy. Flame the loop to incinerate excess bacteria and allow it to cool.Continue to spread the smear with the loop until you obtain an even distribution of organisms over a 2 cm square area and remember to FLAME YOUR LOOP BEFORE PUTTING IT DOWN ON THE BENCH TOP. 3.Allow the smears to air dry(you may speed the drying by holding the slide high above the flame but do not allow them to become hot). 4.Heat fix the dried bacterial smears by passing the glass slides quickly through the flame two or three times. 5.Stain and observe the microscopic morphology and staining characteristics of each microorganism Gram stain Place the slide on the staining rack and stain the films according to the following method: (1)Crystal violet,1-2 minutes (2)Rinse in tap water and blot dry (3)Gram's iodine,1 minute (4)Rinse in tap water and drain excess water (5)Tilt the slide and add acetone-alcohol,drop by drop,until it flows colorlessly (about 5-10 seconds) (6)Rinse in tap water and drain excess water (7)Safranin,30 seconds (8)Rinse in tap water and blot dry (9)Examine with the oil immersion lens and draw the microscopic morphology microscopic morphology file://CWINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (3of 6)[8/3/0024:9:54 PM)
gram-positive and gram-negative bacteria. The difference between these bacteria is that gram positives are not decolorized when alcohol or acetone is applied to the smear. They thus retain the primary stain and are colored purple when observed microscopically under oil immersion. Gram-negative bacteria, however, lose the primary stain upon decolorization and appear pink or red because of the safranin counterstain. >>>>> 1. To prepare a smear from a broth culture (liquid suspension of bacterial cells), merely spread a loopful of the culture over a 2 cm square area of the microscope slide. Aseptic technique must be used to accomplish this transfer and remember to ALWAYS FLAME LOOP BEFORE AND AFTER USE!! 2. For colonies on agar plate, place a loop (or two) of water on a glass slide. Using aseptic technique, gently touch the bacterial surface growth with an inoculating needle or loop and remove a small part of the colony. Emulsify the bacteria on the loop into the drop of water until it just looks cloudy. Flame the loop to incinerate excess bacteria and allow it to cool. Continue to spread the smear with the loop until you obtain an even distribution of organisms over a 2 cm square area and remember to FLAME YOUR LOOP BEFORE PUTTING IT DOWN ON THE BENCH TOP. 3. Allow the smears to air dry (you may speed the drying by holding the slide high above the flame but do not allow them to become hot). 4. Heat fix the dried bacterial smears by passing the glass slides quickly through the flame two or three times. 5. Stain and observe the microscopic morphology and staining characteristics of each microorganism. Gram stain Place the slide on the staining rack and stain the films according to the following method: (1) Crystal violet, 1-2 minutes (2) Rinse in tap water and blot dry (3) Gram's iodine, 1 minute (4) Rinse in tap water and drain excess water (5) Tilt the slide and add acetone-alcohol, drop by drop, until it flows colorlessly (about 5-10 seconds) (6) Rinse in tap water and drain excess water (7) Safranin, 30 seconds (8) Rinse in tap water and blot dry (9) Examine with the oil immersion lens and draw the microscopic morphology microscopic morphology Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (3 of 6) [8/31/2002 4:29:54 PM]

Exercise C.Brightfield Microscopy 1.Compound microscope Since all the living forms(microbes)with which we will be dealing are invisible to the naked eye,a microscope is an essential tool in any laboratory course in microbiology.The compound microscope employs two lens systems:the ocular or eyepiece and the objective lens.These two lens systems are separated so that the ocular lens can magnify the image formed y the objective lens.An object which is magnified 45 times by the high-dry objective lens(45x)is actually magnified 450 times when combined with the magnification provided by the ocular lens which is usually 10x.A bacterial cell with a linear diameter of 0.001 mm(1 u)can thus be magnified to the point of being visible in the microscope(0.001 mm times 450 equals 0.45 mm). The resolving power of the compound microscope is approximately 0.2u (0.0002 mm).This means that objects which are smaller than 0.2 u be distinguished clearly by the lenses and these will appear as small blurs in the field.Increased magnification will only make the blurs larger.Objects which are less than 0.2 u in diameter thus cannot be resolved by the optical microscope. 2.Use and Care of the Microscope a.Never look through eyepiece when turning adjustment knobs downward You should always begin focusing upward.Otherwise,the objective lens will go through the glass slide,thereby scratching and damaging the expensive lenses of the high-dry and oil-immersion objectives. b.Lenses should be cleaned with a lens cleaning solution and lens paper each day before and after use.Xylene can be used to remove any oil film buildup on the lenses,but it should be used sparingly and must be removed by wiping with lens tissue (xylene is a solvent which can dissolve the cement that holds the lens in the objective). 3.Oil immersion Technique a.Place a drop of immersion oil over the specimen that has been previously fixed onto a glass slide b.Using the course adjustment knob,move the oil-immersion objective (100x)into the oil and gently make contact with the slide(watch from side of scope during this procedure). c.Focusing of specimen is accomplished by turning the fine adjustment knob in a direction which makes the objective travel upward! d.If your microscope is parfocal,you can focus a specimen using the high dry objective,and then place a drop of oil on the specimen,switch to the crob Lab We 2001/Exercise1/e> 1b.htm(4of6)[831/20024:29:54PM
C. Brightfield Microscopy 1. Compound microscope Since all the living forms (microbes) with which we will be dealing are invisible to the naked eye, a microscope is an essential tool in any laboratory course in microbiology. The compound microscope employs two lens systems: the ocular or eyepiece and the objective lens. These two lens systems are separated so that the ocular lens can magnify the image formed by the objective lens. An object which is magnified 45 times by the high-dry objective lens (45x) is actually magnified 450 times when combined with the magnification provided by the ocular lens which is usually 10x. A bacterial cell with a linear diameter of 0.001 mm (1 u) can thus be magnified to the point of being visible in the microscope (0.001 mm times 450 equals 0.45 mm). The resolving power of the compound microscope is approximately 0.2u (0.0002 mm). This means that objects which are smaller than 0.2 u cannot be distinguished clearly by the lenses and these will appear as small blurs in the field. Increased magnification will only make the blurs larger. Objects which are less than 0.2 u in diameter thus cannot be resolved by the optical microscope. 2. Use and Care of the Microscope a. Never look through eyepiece when turning adjustment knobs downward. You should always begin focusing upward. Otherwise, the objective lens will go through the glass slide, thereby scratching and damaging the expensive lenses of the high-dry and oil-immersion objectives. b. Lenses should be cleaned with a lens cleaning solution and lens paper each day before and after use. Xylene can be used to remove any oil film buildup on the lenses, but it should be used sparingly and must be removed by wiping with lens tissue (xylene is a solvent which can dissolve the cement that holds the lens in the objective). 3. Oil immersion Technique a. Place a drop of immersion oil over the specimen that has been previously fixed onto a glass slide. b. Using the course adjustment knob, move the oil-immersion objective (100x) into the oil and gently make contact with the slide (watch from side of scope during this procedure). c. Focusing of specimen is accomplished by turning the fine adjustment knob in a direction which makes the objective travel upward! d. If your microscope is parfocal, you can focus a specimen using the high dry objective, and then place a drop of oil on the specimen, switch to the Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (4 of 6) [8/31/2002 4:29:54 PM]