版权所有:华东理工大学物理化学教研室 30 3). Volume, V, m3 4). The amount of substance n, mole (mol) 1.1 The states of gases
版权所有:华东理工大学物理化学教研室 30 3). Volume, V, m3 4). The amount of substance n, mole (mol) 1.1 The states of gases

版权所有:华东理工大学物理化学教研室 31 1). Three important gas Laws 3). Mixtures of gases 2). The combined gas Law 1.2 The gas laws 4). Mole fractions and partial pressures
版权所有:华东理工大学物理化学教研室 31 1). Three important gas Laws 3). Mixtures of gases 2). The combined gas Law 1.2 The gas laws 4). Mole fractions and partial pressures
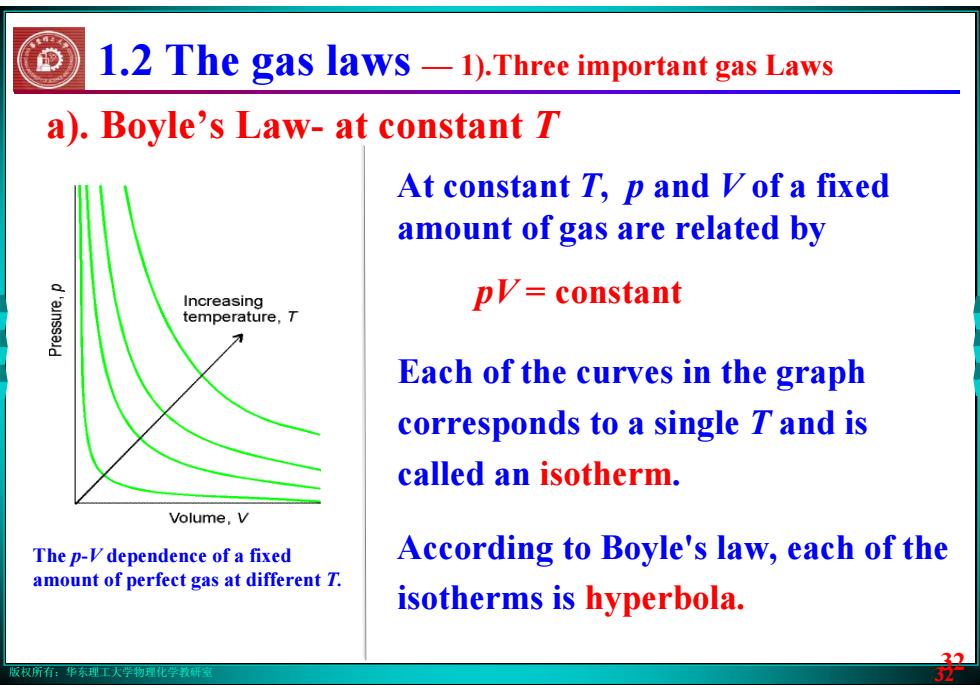
版权所有:华东理工大学物理化学教研室 32 a). Boyle’s Law- at constant T At constant T, p and V of a fixed amount of gas are related by pV = constant Each of the curves in the graph corresponds to a single T and is called an isotherm. According to Boyle's law, each of the isotherms is hyperbola. 1.2 The gas laws — 1).Three important gas Laws The p-V dependence of a fixed amount of perfect gas at different T. 32
版权所有:华东理工大学物理化学教研室 32 a). Boyle’s Law- at constant T At constant T, p and V of a fixed amount of gas are related by pV = constant Each of the curves in the graph corresponds to a single T and is called an isotherm. According to Boyle's law, each of the isotherms is hyperbola. 1.2 The gas laws — 1).Three important gas Laws The p-V dependence of a fixed amount of perfect gas at different T. 32
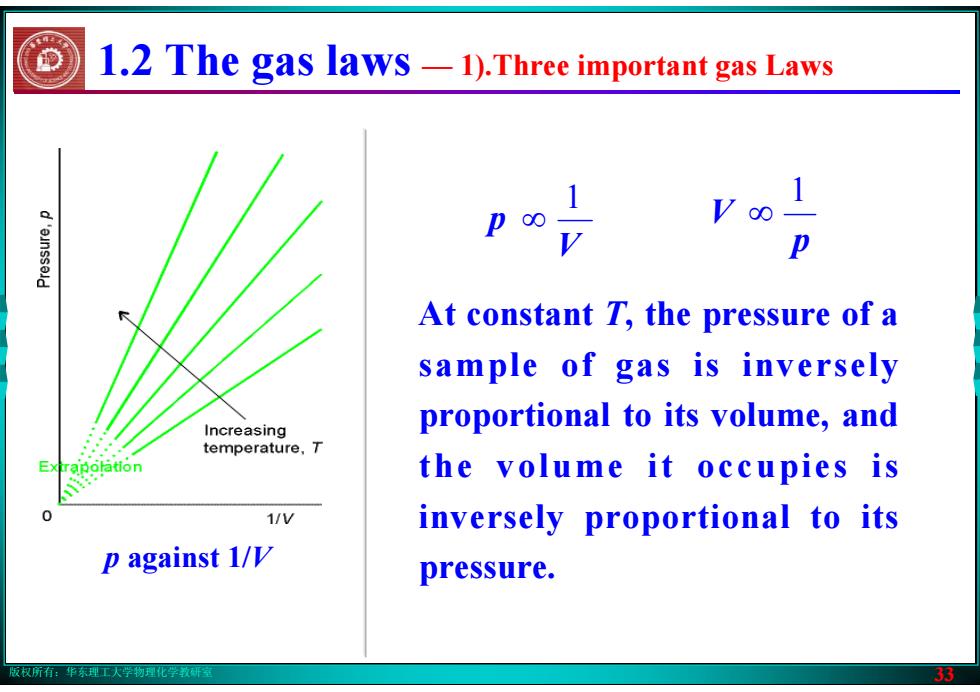
版权所有:华东理工大学物理化学教研室 33 V p 1 ∞ p V 1 ∞ At constant T, the pressure of a sample of gas is inversely proportional to its volume, and the volume it occupies is inversely proportional to its pressure. p against 1/V 1.2 The gas laws — 1).Three important gas Laws
版权所有:华东理工大学物理化学教研室 33 V p 1 ∞ p V 1 ∞ At constant T, the pressure of a sample of gas is inversely proportional to its volume, and the volume it occupies is inversely proportional to its pressure. p against 1/V 1.2 The gas laws — 1).Three important gas Laws

版权所有:华东理工大学物理化学教研室 34 The limiting law Boyle's law is valid only at low pressures;and that real gases obey it only in the limit of the pressure approaching zero ( p →0). Equations that are valid in this limiting sense will be signaled by a º on the equation number in our text book. 1.2 The gas laws — 1).Three important gas Laws
版权所有:华东理工大学物理化学教研室 34 The limiting law Boyle's law is valid only at low pressures;and that real gases obey it only in the limit of the pressure approaching zero ( p →0). Equations that are valid in this limiting sense will be signaled by a º on the equation number in our text book. 1.2 The gas laws — 1).Three important gas Laws