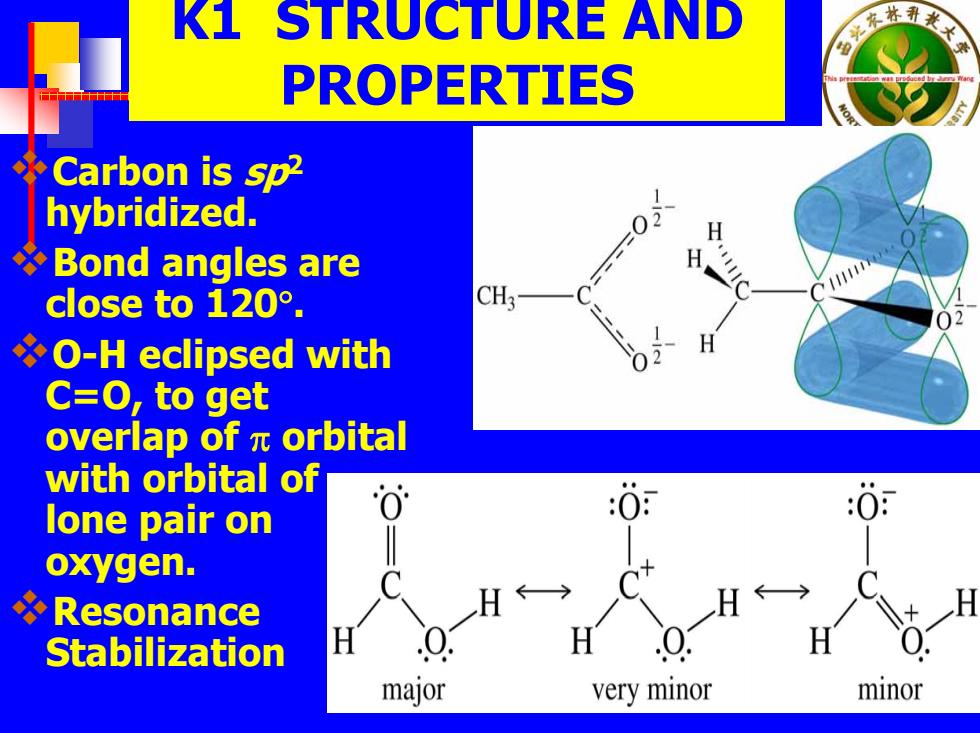
K1 STRUCTURE AND PROPERTIES Carbon is sp2 hybridized. 0) H Bond angles are H 111110 close t0120°. CH O-H eclipsed with C=O,to get overlap ofπorbital with orbital of lone pair on 0 oxygen. Resonance H Stabilization major very minor minor
K1 STRUCTURE AND PROPERTIES Carbon is sp2 hybridized. Bond angles are close to 120 ° . O-H eclipsed with C=O, to get overlap of π orbital with orbital of lone pair on oxygen. Resonance Stabilization
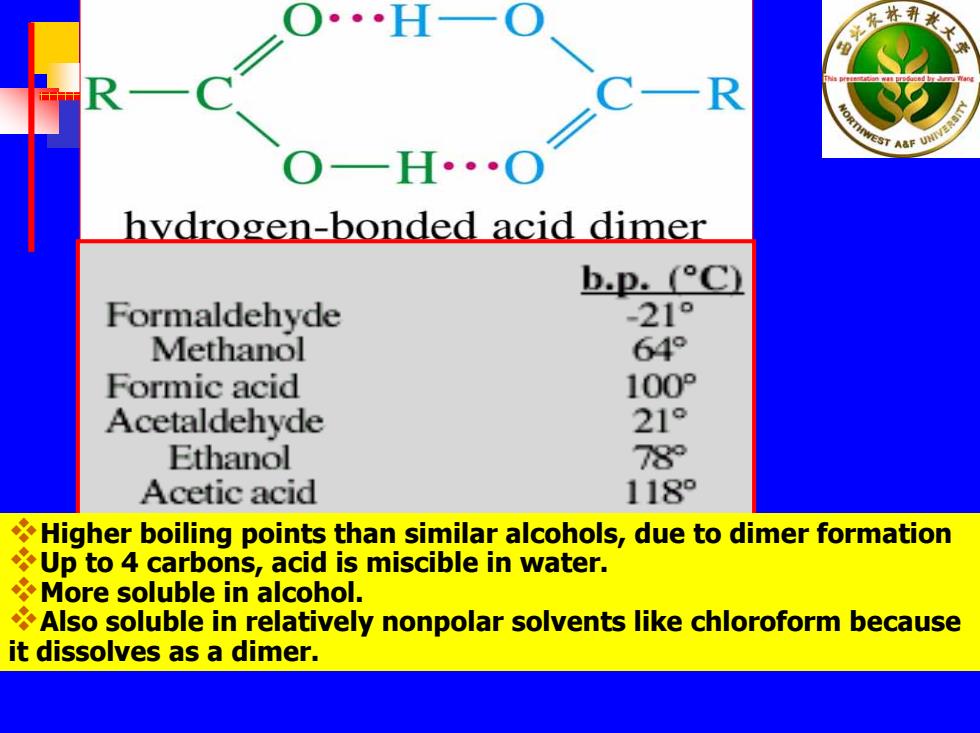
林 R TAaF hydrogen-bonded acid dimer b.p.(C) Formaldehyde -21° Methanol 64° Formic acid 100P Acetaldehyde 21° Ethanol 78 Acetic acid 118 Higher boiling points than similar alcohols,due to dimer formation Up to 4 carbons,acid is miscible in water. More soluble in alcohol. Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer
Higher boiling points than similar alcohols, d ue to dimer form ation Up to 4 carbons, acid is miscible in water. More soluble in alcohol. Also soluble in relativ ely nonpolar solvents like chloroform because it dissolves as a dimer
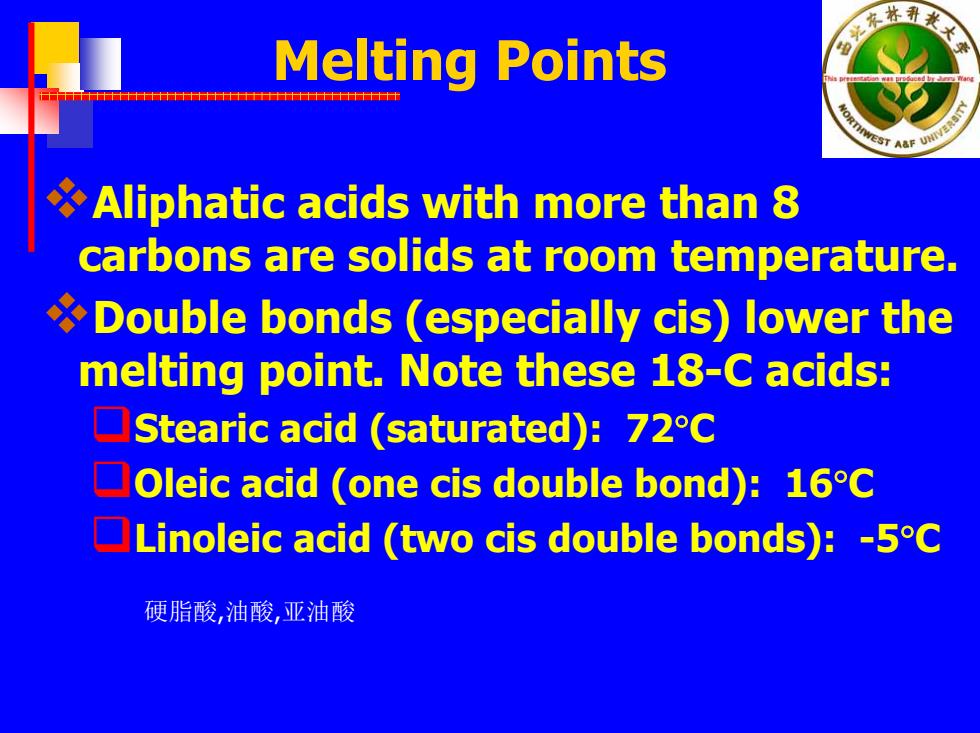
水林 Melting Points AaF0 Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis)lower the melting point.Note these 18-C acids: Stearic acid (saturated):72C Oleic acid (one cis double bond):16C Linoleic acid (two cis double bonds):-5C 硬脂酸,油酸,亚油酸
Melting Points Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis) lower the melting point. Note these 18-C acids: Stearic acid (saturated): 72 ° C Oleic acid (one cis double bond): 16 ° C Linoleic acid (two cis double bonds): -5 ° C 硬脂酸,油酸,亚油酸
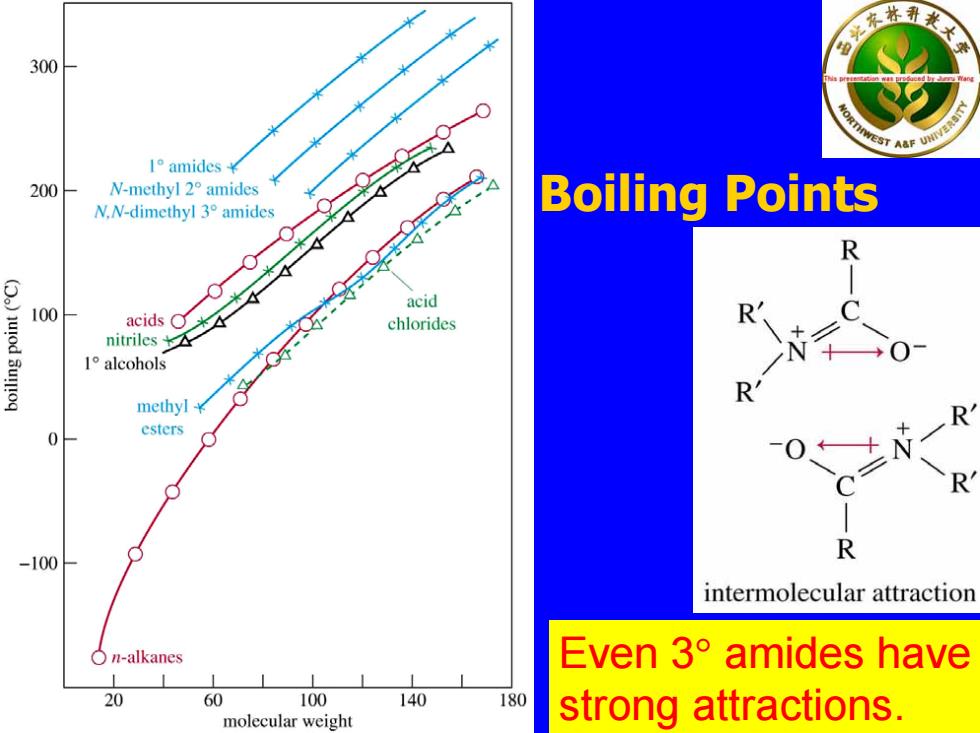
300 EST A&F I°amides¥ 200 N-methyl2°amides N.N-dimethyl 3amides Boiling Points R )uod 3u!!oq acid 100 acids O chlorides R nitriles 1°alcohols methyl¥ esters 0 -100 ● R intermolecular attraction On-alkanes Even3°amides have 20 60 100 140 180 molecular weight strong attractions
Boiling Points Even 3 ° amides have strong attractions
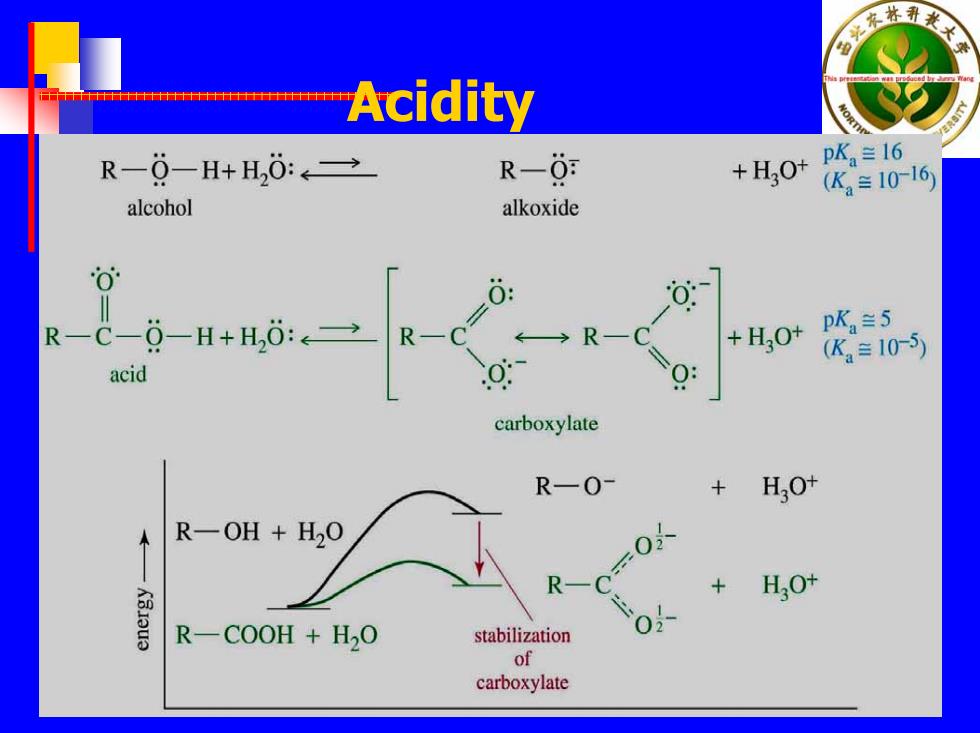
林孔 Acidity R-0-H+H,0:→ R-0: +H,O+ pKa兰16 (K2=10-16) alcohol alkoxide 0 0: R— C-0-H+H,0:→ R-C pKa=5 acid =心 +HO+ (K≡105) carboxylate R一O- + H3O+ R-OH +H2O R- + HO+ K3Iou3 R-COOH +H2O stabilization of carboxylate
Acidity