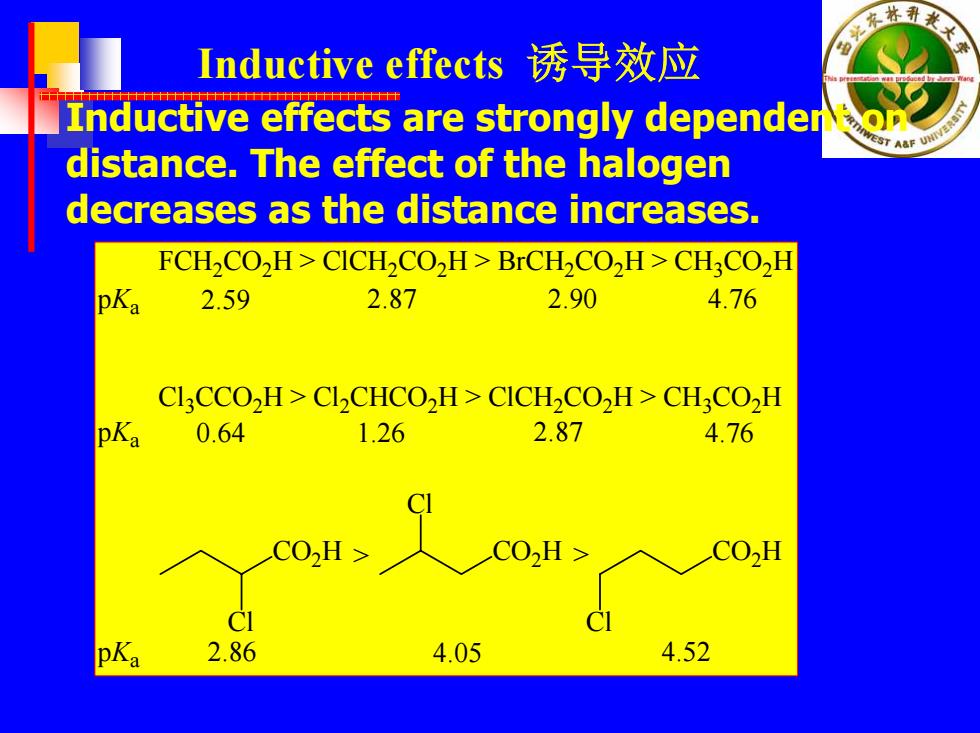
Inductive effects诱导效应 Inductive effects are strongly depender EST A&F distance.The effect of the halogen decreases as the distance increases. FCHCO,H>CICH2CO,H>BrCH,CO,H>CH:CO,H pKa 2.59 2.87 2.90 4.76 CICCO,H>CI2CHCO2H>CICH2CO2H>CHCO2H pKa 0.64 1.26 2.87 4.76 人m,H>义 C02H> CO,H pKa 2.86 4.05 4.52
Inductive effects 诱导效应 FC H 2CO 2H > C l C H 2CO 2H > B r C H 2CO 2H > C H 3CO 2 H Cl 3CCO 2H > C l 2CHCO 2H > C l C H 2CO 2H > C H 3CO 2 H CO 2 H Cl CO 2 H Cl CO 2 H Cl > > p Ka p Ka p Ka 2.59 2.87 2.90 4.76 0.64 1.26 2.87 4.76 2.86 4.05 4.52 Inductive effects are strongly dependent on distance. The effect of the halogen decreases as the distance increases
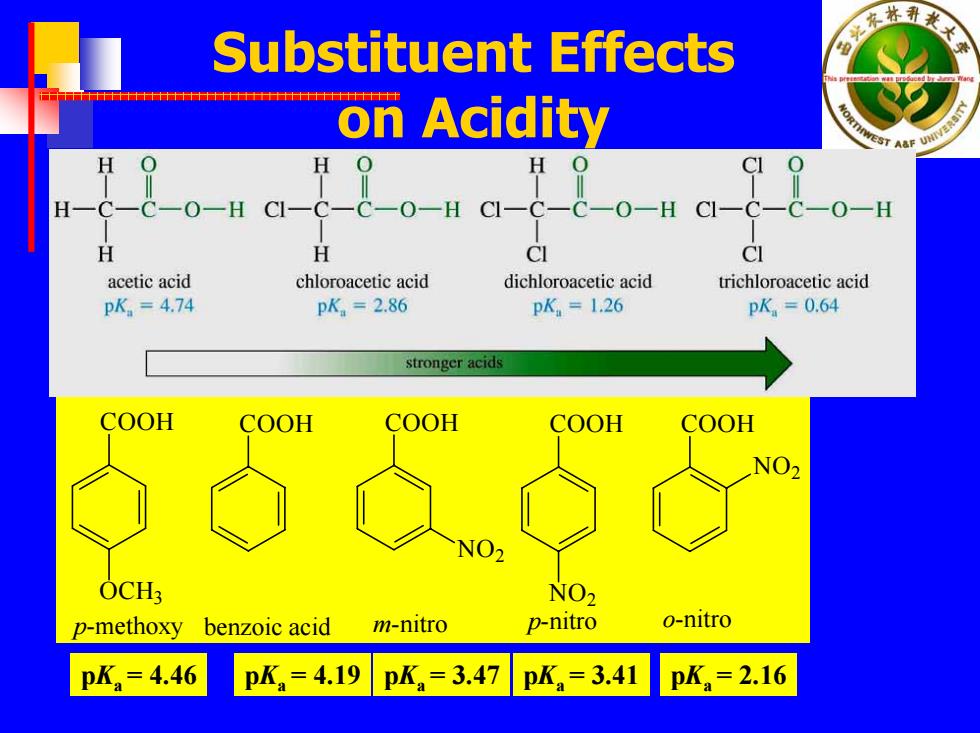
林 Substituent Effects on Acidity 0 H O一H O-H CI- C-C一O-H H H CI CI acetic acid chloroacetic acid dichloroacetic acid trichloroacetic acid pK.=4.74 pK.=286 pK,=1.26 pK=0.64 stronger acids COOH COOH COOH COOH COOH OCH NO p-methoxy benzoic acid m-nitro p-nitro o-nitro pK=4.46 pKa=4.19 pKa=3.47 pK,=3.41 pK=2.16
Substituent Effects on Acidity COOH OC H 3 COOH COOH NO 2 COOH NO 2 COOH NO 2 p-methoxy benzoic acid m-nitro p-nitro o-nitro p Ka = 4.46 p Ka = 4.19 p Ka = 3.47 p Ka = 3.41 p Ka = 2.16
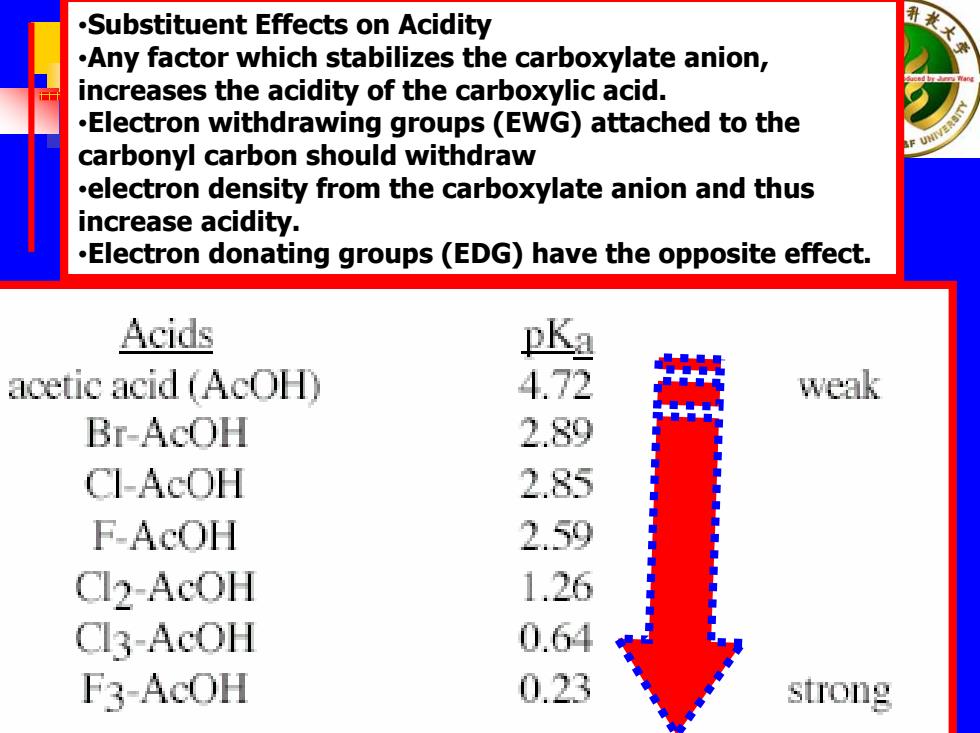
.Substituent Effects on Acidity .Any factor which stabilizes the carboxylate anion, increases the acidity of the carboxylic acid. .Electron withdrawing groups(EWG)attached to the carbonyl carbon should withdraw .electron density from the carboxylate anion and thus increase acidity. .Electron donating groups(EDG)have the opposite effect. Acids pKa acetic acid (AcOH 4.72 weak Br-AcOH 2.89 CI-AcOH 2.85 F-AcOH 2.59 Cl2-AcOH 1.26 Cl3-AcOH 0.64 F3-AcOH 0.23 strong
•Substituent Effects on Acidity •Any factor which stabilizes the carboxylate anion, increases the acidity of the carboxylic acid. •Elect ron withdrawing groups (EWG) attached to the carbonyl carbon should withdraw •electron density from the carboxylate anion and thus increase acidity. •Elect ron donating groups (EDG) have the opposite effect
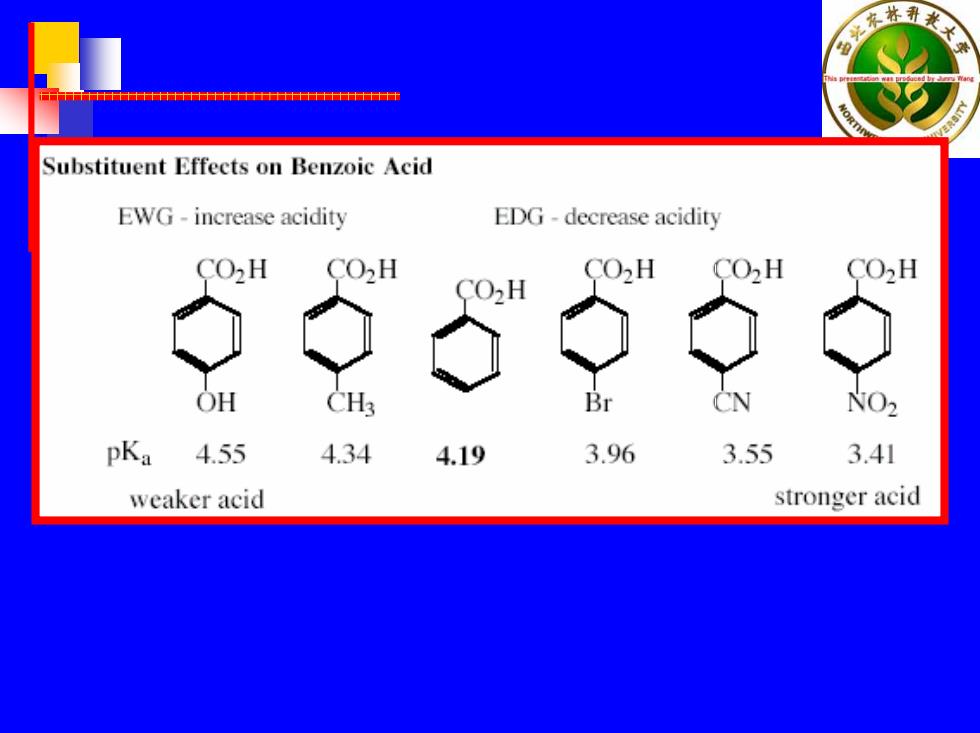
Substituent Effects on Benzoic Acid EWG-increase acidity EDG-decrease acidity CO2H CO2H CO2H CO2H CO2H CO2H OH CH3 Br CN NO2 pKa 4.55 4.34 4.19 3.96 3.55 3.41 weaker acid stronger acid
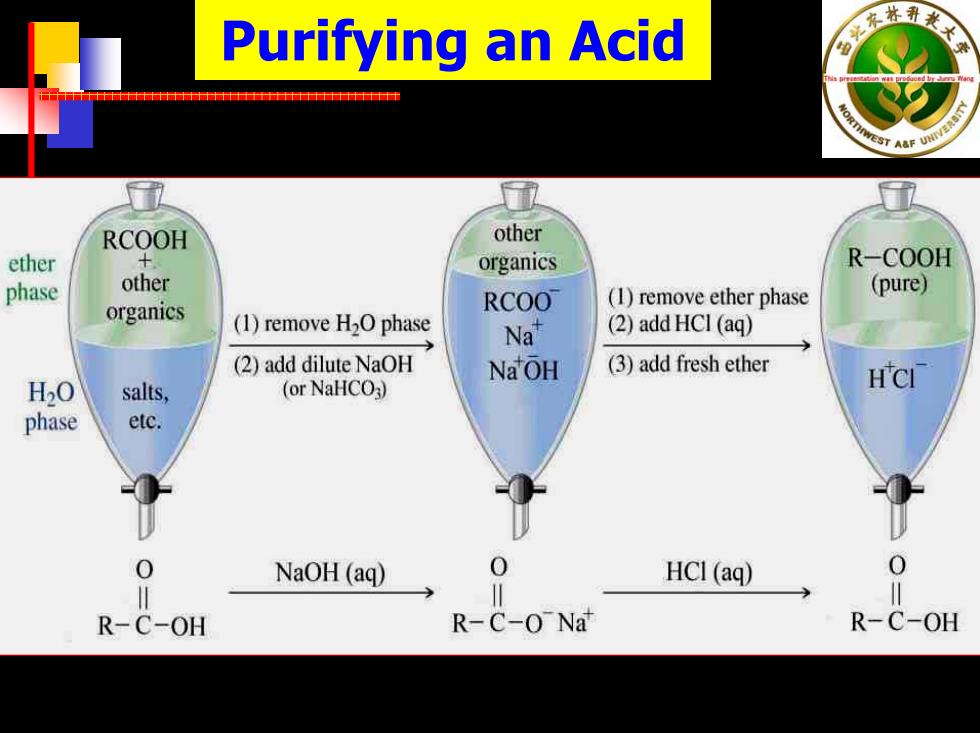
Purifying an Acid 衣林 十十t S7AaF四 RCOOH other ether 十 organics R-COOH phase other (1)remove ether phase (pure) organics RCOO (1)remove H2O phase Na (2)add HCI(aq) (2)add dilute NaOH NaOH (3)add fresh ether H20 HCI salts, (or NaHCO3) phase etc. 0 NaOH (aq) 0 HCI(aq) 0 R-C-OH R-C-0 Nat R-C-OH
Purifying an Acid