
1 Did the tetrahedral coordination compound with different spatial arrangement? no Did the octahedral coordination compound with different spatial arrangement? yes
① Did the tetrahedral coordination compound with different spatial arrangement? no ② Did the octahedral coordination compound with different spatial arrangement? yes

11.1.2 Magnetism of coordination compounds Magnetisms(磁):物质在磁场中表现出来的性质 A moving electrical charge (i.e.,electric current) induces a magnetic field in a material. In an atom,the magnetic field is due to the coupled orbital and spin magnetic moments associated with the motion of electrons.The orbital magnetic moment is due to the motion of electrons around the nucleus,whereas the spin magnetic moment is due to the precession of the electrons about their own axes. The resultant of the orbital and spin magnetic moments of the constituent atoms of a material gives rise to the observed magnetic properties
11.1.2 Magnetism of coordination compounds Magnetisms( 磁):物质在磁场中表现出来的性质 A moving electrical charge (i.e., electric current) induces a magnetic field in a material. In an atom, the magnetic field is due to the coupled orbital and spin magnetic moments associated with the motion of electrons. The orbital magnetic moment is due to the motion of electrons around the nucleus, whereas the spin magnetic moment is due to the precession of the electrons about their own axes. The resultant of the orbital and spin magnetic moments of the constituent atoms of a material gives rise to the observed magnetic properties
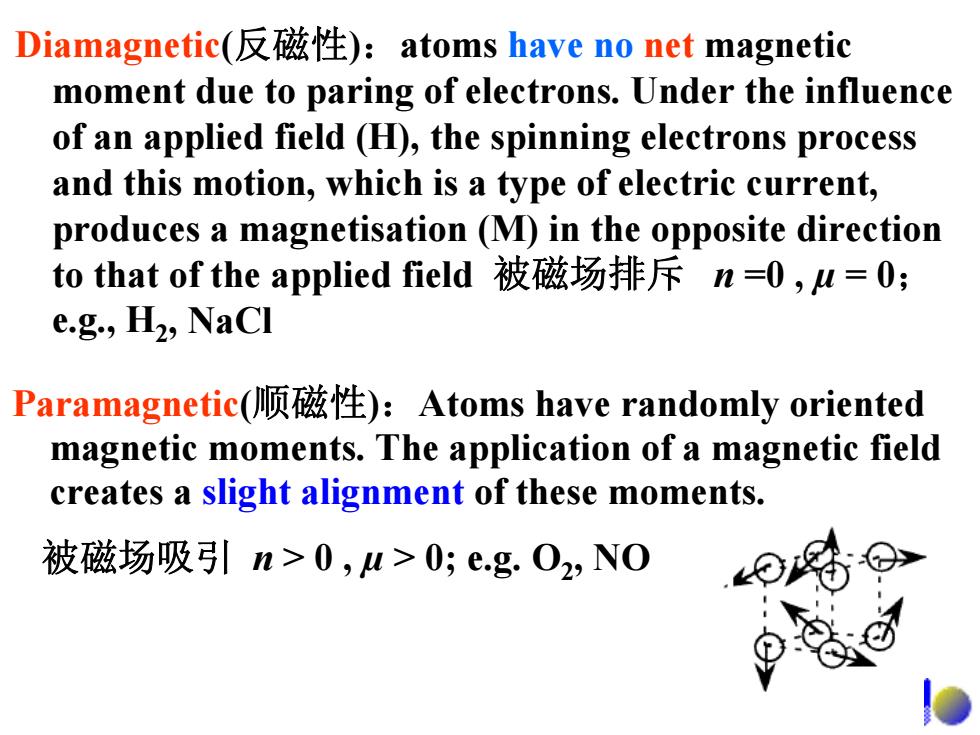
Diamagnetic(反磁性):atoms have no net magnetic moment due to paring of electrons.Under the influence of an applied field (H),the spinning electrons process and this motion,which is a type of electric current, produces a magnetisation(M)in the opposite direction to that of the applied field被磁场排斥n=0,u=0; e.g.,H2,NaCI Paramagnetic(顺磁性):Atoms have randomly oriented magnetic moments.The application of a magnetic field creates a slight alignment of these moments. 被磁场吸引n>0,u>0;e.g.O2,N0
Paramagnetic(顺磁性):Atoms have randomly oriented magnetic moments. The application of a magnetic field creates a slight alignment of these moments. 被磁场吸引 n > 0 , µ > 0; e.g. O2, NO Diamagnetic(反磁性):atoms have no net magnetic moment due to paring of electrons. Under the influence of an applied field (H), the spinning electrons process and this motion, which is a type of electric current, produces a magnetisation (M) in the opposite direction to that of the applied field 被磁场排斥 n =0 , µ = 0; e.g., H2, NaCl

Ferromagnetic(铁磁性):Atoms have parallel aligned magnetic moments被磁场强烈吸引。E.g:Fe,Co,Ni Antiferromanetic(反铁磁性 Ferromagneic Atoms have mixed parall 铁磁性 and anti-parallel aligned 梦® magnetic moments Antiferromagnetic 中甲 反铁磁性 Ferrimagnetic(亚铁磁性): Atoms have anti-parallel Ferrimagnetic aligned magnetic moment 亚铁磁性 of different magnitude
Ferrimagnetic(亚铁磁性): Atoms have anti-parallel aligned magnetic moments of different magnitude Ferromagnetic( 铁磁性):Atoms have parallel aligned magnetic moments被磁场强烈吸引。E.g:Fe,Co,Ni Antiferromanetic(反铁磁性): Atoms have mixed parallel and anti-parallel aligned magnetic moments Ferromagneic 铁磁性 Antiferromagnetic 反铁磁性 Ferrimagnetic 亚铁磁性
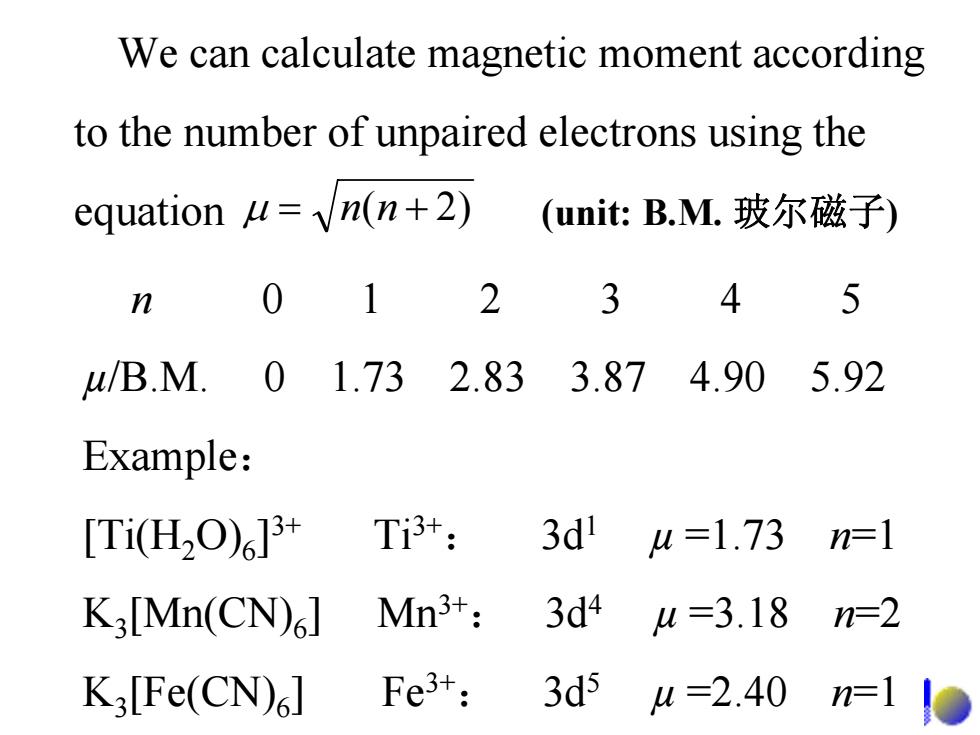
We can calculate magnetic moment according to the number of unpaired electrons using the equation u=n(n+2) (unit:B.M.玻尔磁子) n 2 3 u/B.M. 01.732.833.874.90 5.92 Example: [Ti(H2O)6]3+ Ti3+: 3d' u=1.73 n=1 K3[Mn(CN)] Mn3+: 3d4 u=3.18 n=2 K3[Fe(CN).] Fe3+: 3d5 4=2.40 n=1
n 0 1 2 3 4 5 µ/B.M. 0 1.73 2.83 3.87 4.90 5.92 Example: [Ti(H2O)6]3+ Ti3+: 3d1 µ =1.73 n=1 K3[Mn(CN)6] Mn3+: 3d4 µ =3.18 n=2 K3[Fe(CN)6] Fe3+: 3d5 µ =2.40 n=1 μ nn += )2( We can calculate magnetic moment according to the number of unpaired electrons using the equation (unit: B.M. 玻尔磁子)