
第二节 硫醇和硫酚 e 硫醇和硫醚制备 RX KHS RSH +KX C=CH2 +H2S H2S04 (CH3)3C-SH CH3 H一S: D R-SH X Use a large excess of sodium hydrosulfide with unhindered alkyl halide to prevent dialkylation to R-S-R
一、硫醇和硫醚制备 RX + KHS RSH + KX C CH2 CH3 CH3 + H2 S H2 SO4 (CH3 ) 3 C SH 第二节 硫醇和硫酚 Use a large excess of sodium hydrosulfide with unhindered alkyl halide to prevent dialkylation to R-S-R. H S _ R X R SH + _ X
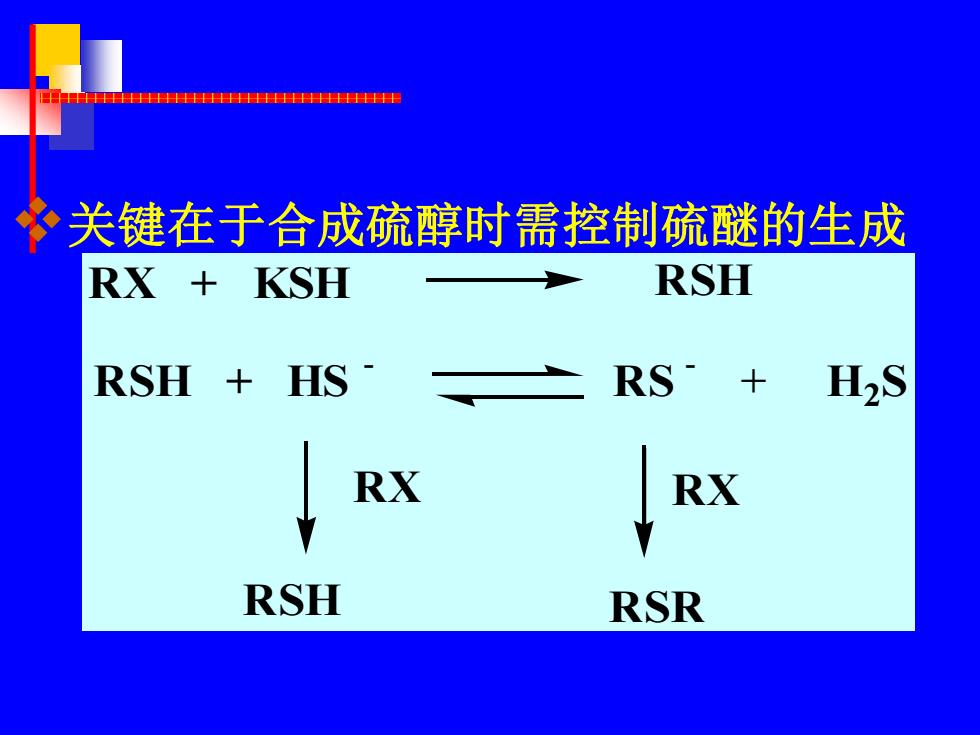
P 关键在于合成硫醇时需控制硫醚的生成 RX+ KSH RSH RSH HS RS H2S RX RX RSH RSR
❖关键在于合成硫醇时需控制硫醚的生成 RX + KSH RSH RSH + HS - RS - + H2 S R X RSH R X RSR

硫醚制备 2RX Na2S RSR 2NaX A sulfide NaOH RX RSH -RSNa RSR CI+Na2S + 2Na"CI 1,4-Dichlorobutane Thiolane (Tetrahydrothiophene)
硫醚制备 A sulfide 2 RX + Na2 S RSR + 2 NaX S N2 Thiolane (Tetrahydrothiophene) 1,4-Dichlorobutane + Na2 S + S 2 Na + Cl - Cl Cl RSH RSNa RSR' NaOH R'X

P CH3(CH2)8CH2S Na*CHgl Sodium 1-decanethiolate CH3(CH28CH2SCH3 Na*r 1-Methylsulfanyldecane (Decyl methyl sulfide)
SN 2 Sodium 1-decanethiolate + + CH3 (CH2 ) 8 CH2 S - Na + CH3 I CH3 (CH2 ) 8 CH2 SCH3 Na + I - 1-Methylsulfanyldecane (Decyl methyl sulfide)
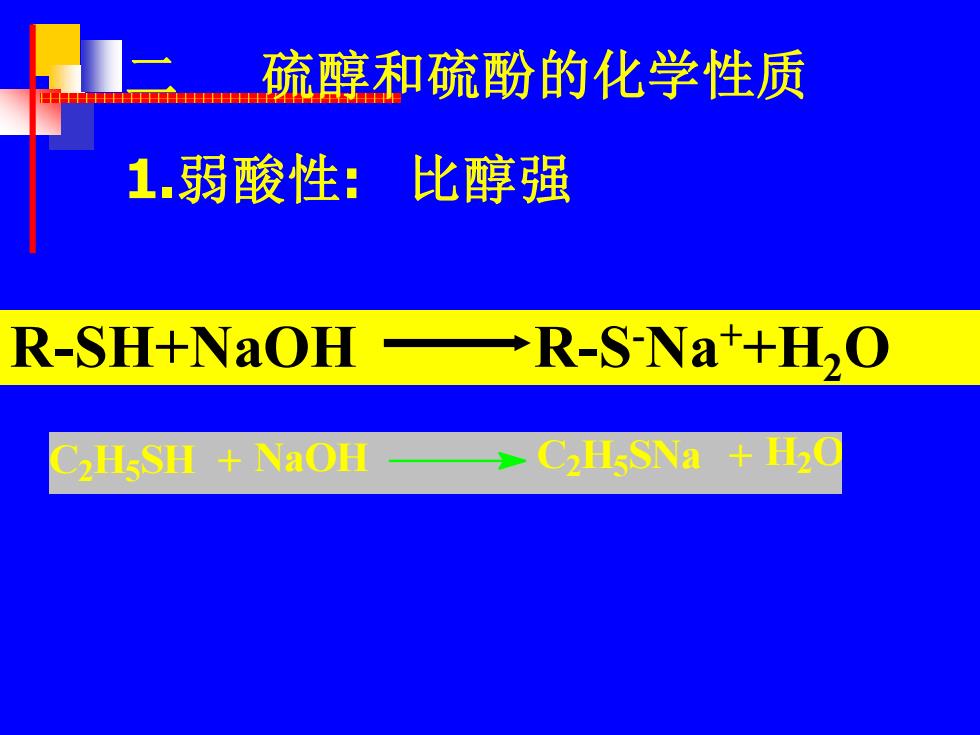
硫醇和硫酚的化学性质 1.弱酸性:比醇强 R-SH+NaOH R-S-Na+H,O C2H5SH NaOH C2H5SNa H2C
1.弱酸性: 比醇强 二 硫醇和硫酚的化学性质 R-SH+NaOH R-S -Na++H2O C2 H5 SH + NaOH C2 H5 SNa + H2 O