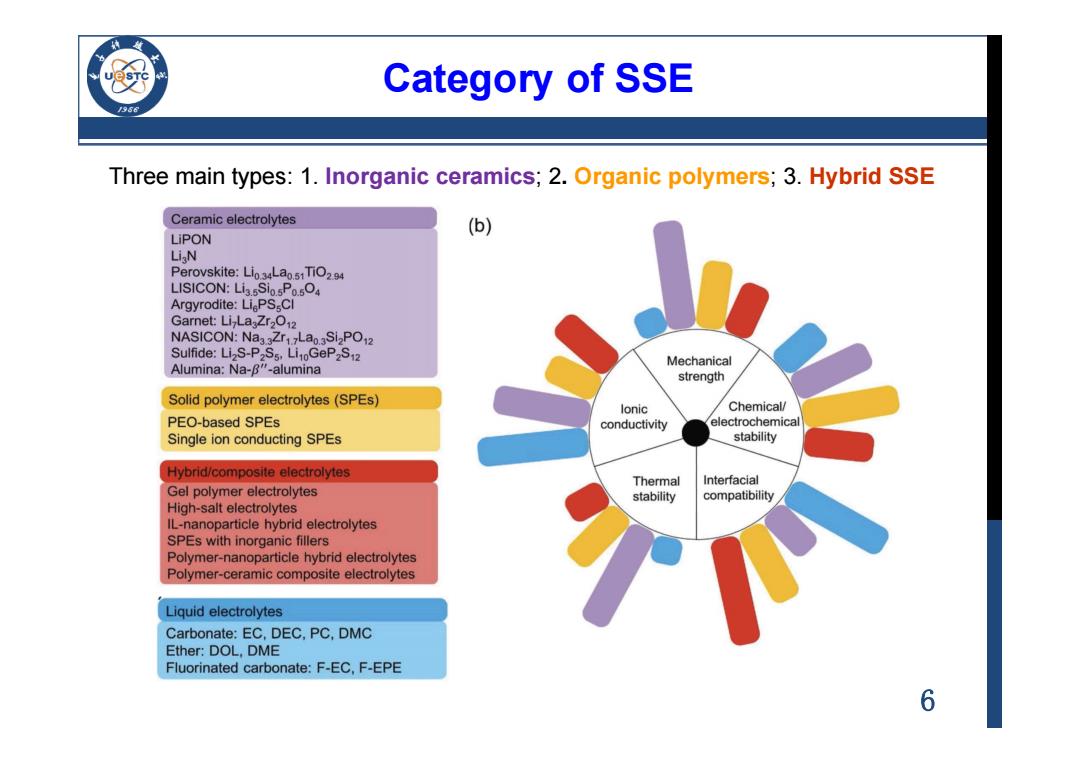
Category of SSE /956 Three main types:1.Inorganic ceramics;2.Organic polymers;3.Hybrid SSE Ceramic electrolytes (b) LiPON Li N Perovskite:Lio4Lao.51TiO24 LISICON:LissSiosPo.sO4 Argyrodite:LiPS CI Garnet:Li-LaZr2O12 NASICON:Na3.3Zr1.Lao.3Si2PO12 Sulfide:Li S-P2Ss.Li0GeP2S12 Mechanical Alumina:Na-B"-alumina strength Solid polymer electrolytes(SPEs) lonic Chemical/ PEO-based SPEs conductivity electrochemical Single ion conducting SPEs stability Hybrid/composite electrolytes Thermal Interfacial Gel polymer electrolytes stability compatibility High-salt electrolytes IL-nanoparticle hybrid electrolytes SPEs with inorganic fillers Polymer-nanoparticle hybrid electrolytes Polymer-ceramic composite electrolytes Liquid electrolytes Carbonate:EC,DEC,PC,DMC Ether:DOL,DME Fluorinated carbonate:F-EC,F-EPE 6
6 Category of SSE Three main types: 1. Inorganic ceramics; 2. Organic polymers; 3. Hybrid SSE
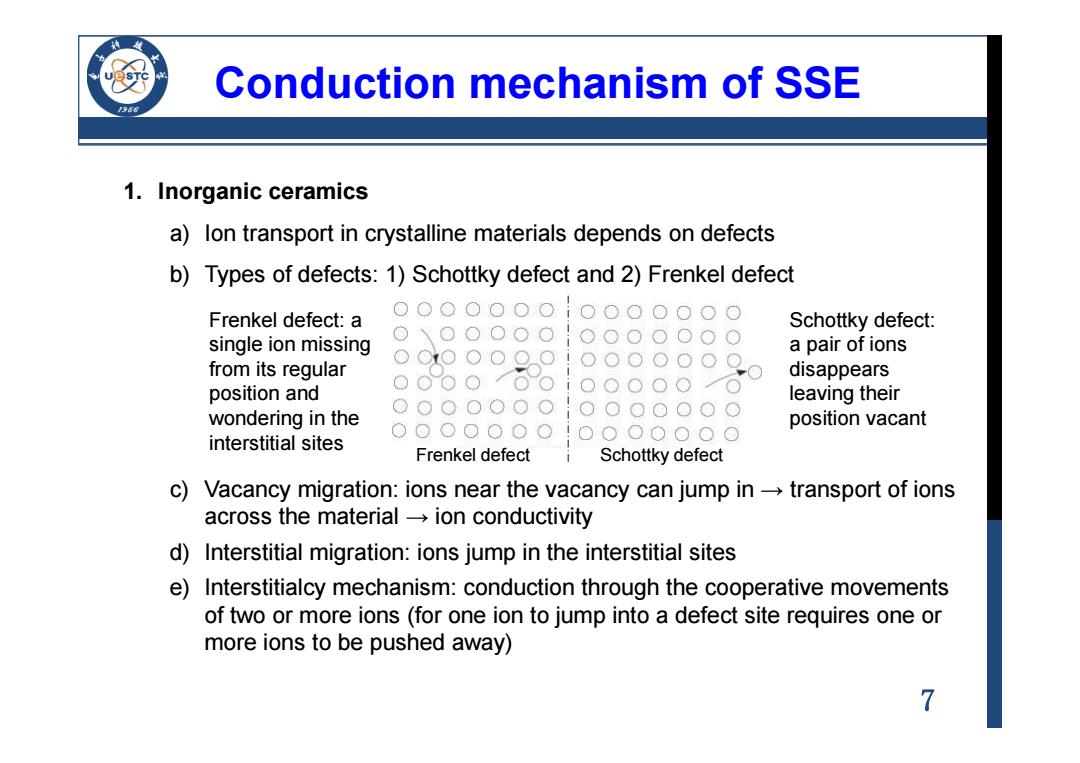
Conduction mechanism of SSE 1.Inorganic ceramics a)lon transport in crystalline materials depends on defects b)Types of defects:1)Schottky defect and 2)Frenkel defect Frenkel defect:a Schottky defect: single ion missing a pair of ions from its regular disappears position and leaving their wondering in the position vacant interstitial sites Frenkel defect Schottky defect c)Vacancy migration:ions near the vacancy can jump in-transport of ions across the materialion conductivity d)Interstitial migration:ions jump in the interstitial sites e)Interstitialcy mechanism:conduction through the cooperative movements of two or more ions (for one ion to jump into a defect site requires one or more ions to be pushed away) 7
7 Conduction mechanism of SSE 1. Inorganic ceramics a) Ion transport in crystalline materials depends on defects b) Types of defects: 1) Schottky defect and 2) Frenkel defect c) Vacancy migration: ions near the vacancy can jump in → transport of ions across the material → ion conductivity d) Interstitial migration: ions jump in the interstitial sites e) Interstitialcy mechanism: conduction through the cooperative movements of two or more ions (for one ion to jump into a defect site requires one or more ions to be pushed away) Frenkel defect Schottky defect Frenkel defect: a single ion missing from its regular position and wondering in the interstitial sites Schottky defect: a pair of ions disappears leaving their position vacant