
Lecture 9 196 Anode material for LIBs: Tin Chen Junsong School of Materials and Energy 2020.04
Anode material for LIBs: Tin Chen Junsong School of Materials and Energy 2020.04 Lecture 9
Content /986 Introduction of Tin 。 Chemistry of Tin Molecular structure and Li insertion Synthesis and modification methods One literature example 2
2 Content • Introduction of Tin • Chemistry of Tin • Molecular structure and Li insertion • Synthesis and modification methods • One literature example
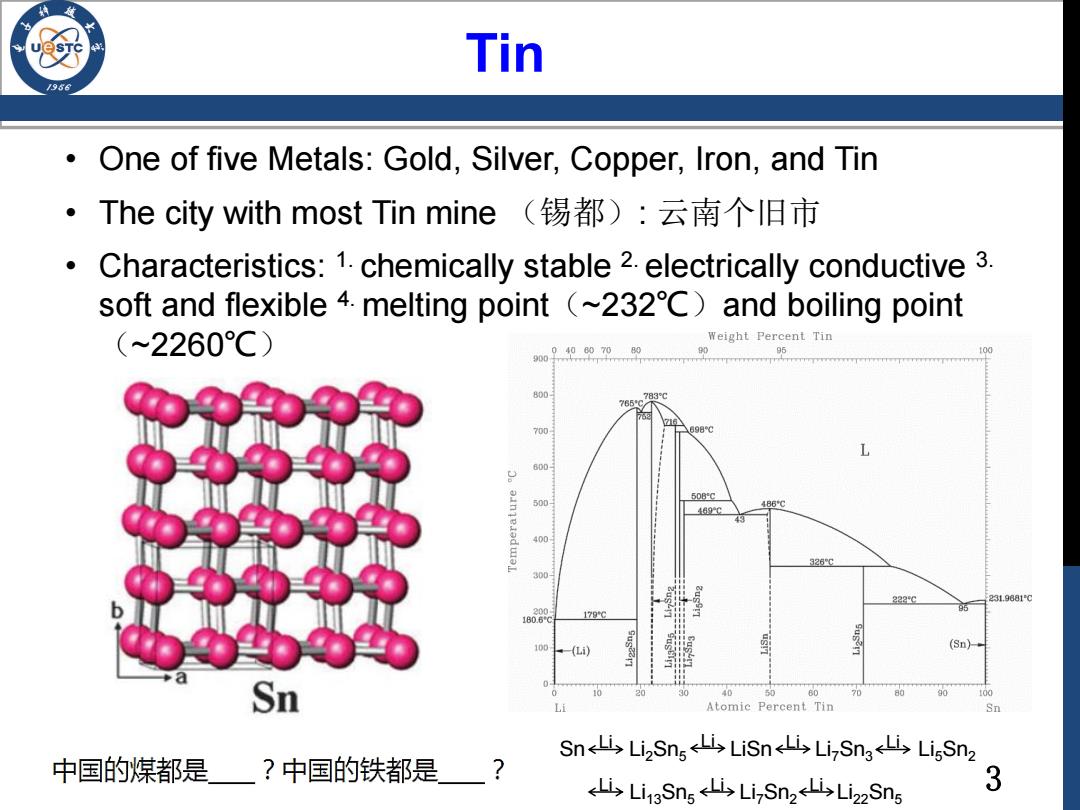
Tin One of five Metals:Gold,Silver,Copper,Iron,and Tin ·The city with most Tin mine(锡都):云南个旧市 Characteristics:1.chemically stable 2.electrically conductive 3. soft and flexible 4.melting point (~232C)and boiling point (~2260℃) Weight Percent Tin 9009406070 95 765 700 698C L 500 50BC 469c 486℃ 326 222C9 231.9081℃ 179G 10D (Sn)- Sn 40 60 100 Li Atomic Percent Tin Sn Sn<4Li2Sn,山LiSn<山Li,Sn3iLi5Snz 中国的煤都是?中国的铁都是 ? 山Li3Sne5 Lis Li,Sn2山Li2zSns 3
3 Tin Sn Li2Sn5 LiSn Li7Sn3 Li5Sn2 Li13Sn5 Li7Sn2 Li22Sn5 Li Li Li Li Li Li Li • One of five Metals: Gold, Silver, Copper, Iron, and Tin • The city with most Tin mine (锡都): 云南个旧市 • Characteristics: 1. chemically stable 2. electrically conductive 3. soft and flexible 4. melting point(~232℃)and boiling point (~2260℃)
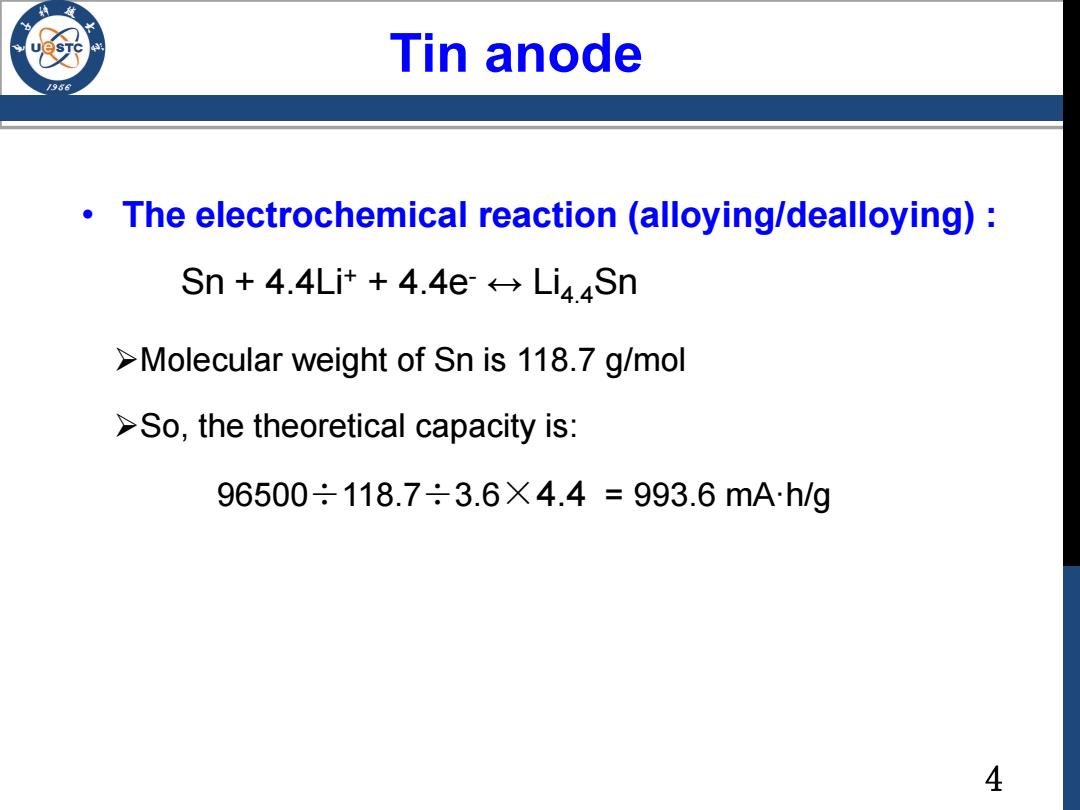
Tin anode /986 The electrochemical reaction (alloying/dealloying): Sn 4.4Li++4.4e Li4Sn >Molecular weight of Sn is 118.7 g/mol >So,the theoretical capacity is: 96500÷118.7÷3.6X4.4=993.6mAh/g 4
4 • The electrochemical reaction (alloying/dealloying) : Sn + 4.4Li+ + 4.4e- ↔ Li4.4Sn Molecular weight of Sn is 118.7 g/mol So, the theoretical capacity is: 96500÷118.7÷3.6×4.4 = 993.6 mA·h/g Tin anode
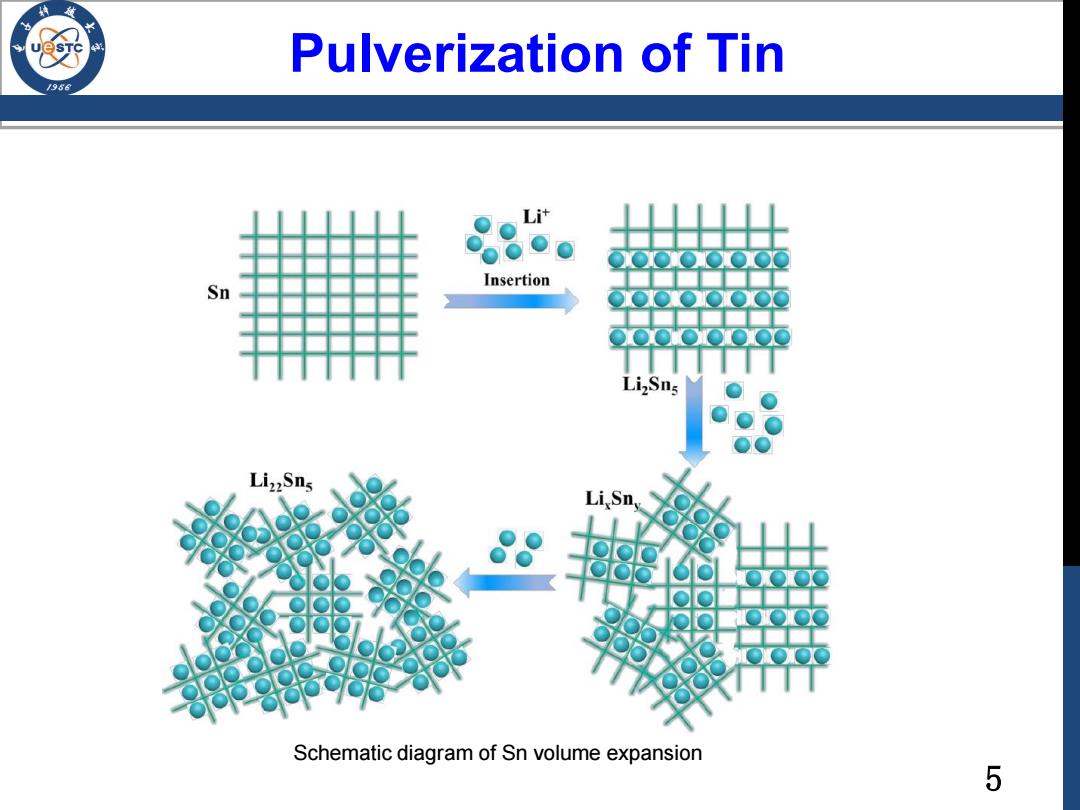
Pulverization of Tin /96 L Sn Insertion Li2Sns Liz2Sns Li Sn Schematic diagram of Sn volume expansion 5
5 Pulverization of Tin Schematic diagram of Sn volume expansion