
Lecture 6 196 Anode material for LIBs: Graphite Chen Junsong School of Materials and Energy 2020.04
Anode material for LIBs: Graphite Chen Junsong School of Materials and Energy 2020.04 Lecture 6
Content /986 Overview of anode materials Introduction of graphite 。 Chemistry of graphite Molecular structure and Li insertion Synthesis and modification methods One literature example 2
2 Content • Overview of anode materials • Introduction of graphite • Chemistry of graphite • Molecular structure and Li insertion • Synthesis and modification methods • One literature example

Overview /986 。 General requirement React with Li+at a low potential vs.Li/Lit With a high Lit storage capacity Category:storage mechanism Insertion/deinsertion:graphite,TiO2,etc. Alloy/dealloy:Sn,Tin,etc. Replacement reaction:Fe2O3,Co3O4,etc. 3
3 Overview • General requirement React with Li+ at a low potential vs. Li/Li+ With a high Li+ storage capacity • Category: storage mechanism • Insertion/deinsertion: graphite, TiO2 , etc. • Alloy/dealloy: Sn, Tin, etc. • Replacement reaction: Fe2O3 , Co3O4 , etc
Graphite /98 Crystalline allotrope of carbon,most stable form Characteristics:1.thermally and chemically stable 2.thermally and electrically conductive 3.lubricating Origin:graphite mines in Heilongjiang,Shandong Preparation:purification of graphite mine: >1)chemical:NaOH reaction neutralize using HCI >2)physical:annealing at high T without oxygen https://b2b.hc360.com/viewPics/s https://b2b.hc360.com/viewPics/s http://www.99114.com/pi upplyself_pics/80332485791.html upplyself_pics/80332485791.html cture/598650.html 4
http://www.99114.com/pi cture/598650.html https://b2b.hc360.com/viewPics/s upplyself_pics/80332485791.html https://b2b.hc360.com/viewPics/s upplyself_pics/80332485791.html 4 Graphite • Crystalline allotrope of carbon, most stable form • Characteristics: 1. thermally and chemically stable 2. thermally and electrically conductive 3. lubricating • Origin:graphite mines in Heilongjiang, Shandong • Preparation:purification of graphite mine: 1) chemical:NaOH reaction → neutralize using HCl 2) physical:annealing at high T without oxygen
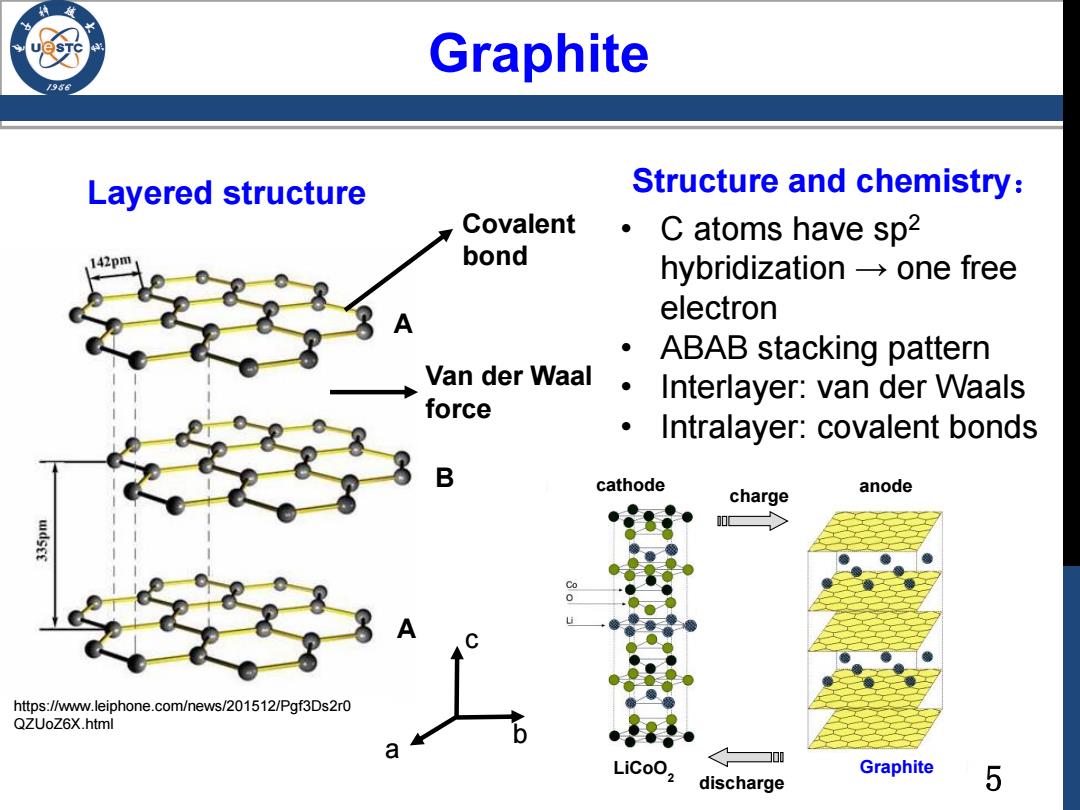
Graphite Layered structure Structure and chemistry: Covalent C atoms have sp2 42pm bond hybridization-one free electron ABAB stacking pattern Van der Waal 。 Interlayer:van der Waals force Intralayer:covalent bonds B cathode charge anode https://www.leiphone.com/news/201512/Pgf3Ds2r0 QZUoZ6X.html a LiCoO2 Graphite discharge 5
5 • C atoms have sp2 hybridization → one free electron • ABAB stacking pattern • Interlayer: van der Waals • Intralayer: covalent bonds A B A https://www.leiphone.com/news/201512/Pgf3Ds2r0 QZUoZ6X.html Layered structure Covalent bond Van der Waal force Structure and chemistry: charge discharge cathode LiCoO2 Graphite anode Graphite a b c