
Sec 1 Preparing Alkyl Halides from Alkenes: Allylic Bromination Products of allylic bromination reactions are useful for conversion into conjugated dienes by dehydrohalogenation with base Br hv NBS KOH CCl4 Cyclohexene 3-Bromocyclohexene Cyclohexa-1,3-diene
▪ Products of allylic bromination reactions are useful for conversion into conjugated dienes by dehydrohalogenation with base Sec 1 Preparing Alkyl Halides from Alkenes: Allylic Bromination

Worked Example Predicting the Product of an Allylic Bromination Reaction What products would you expect from the reaction of 4,4-dimethylcyclohexene with NBS?
What products would you expect from the reaction of 4,4-dimethylcyclohexene with NBS? Worked Example Predicting the Product of an Allylic Bromination Reaction

Worked Example Predicting the Product of an Allylic Bromination Reaction Strategy Draw the alkene reactant and identify the allylic positions. Label the two different allylic positions A and B.Now abstract an allylic hydrogen from each position to generate the two corresponding allylic radicals.Each of the two allylic radicals can add a Br atom at either end(A or a;B or b)to give a mixture of up to four products.Draw and name the products
Strategy Draw the alkene reactant and identify the allylic positions. Label the two different allylic positions A and B. Now abstract an allylic hydrogen from each position to generate the two corresponding allylic radicals. Each of the two allylic radicals can add a Br atom at either end (A or a; B or b) to give a mixture of up to four products. Draw and name the products Worked Example Predicting the Product of an Allylic Bromination Reaction
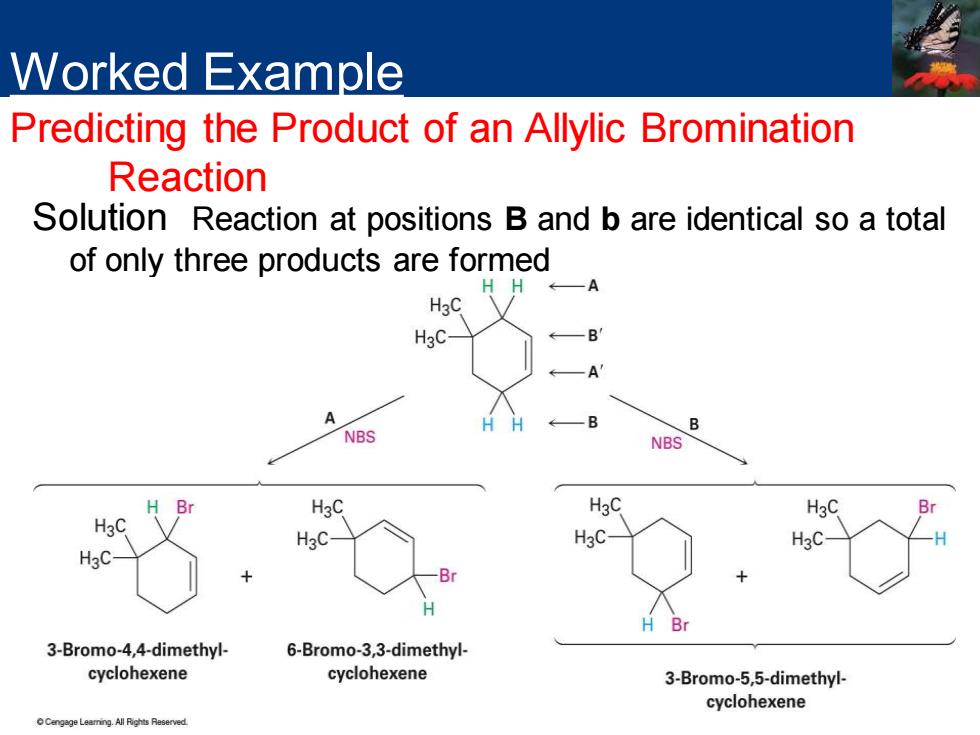
Worked Example Predicting the Product of an Allylic Bromination Reaction Solution Reaction at positions B and b are identical so a total of only three products are formed HH←A H3C H3C -B —A H ←—B B NBS NBS H Br H3C H3C H3C B H3C H3C- H3C- H3C B Br 3-Bromo-4,4-dimethyl- 6-Bromo-3,3-dimethyl- cyclohexene cyclohexene 3-Bromo-5,5-dimethyl- cyclohexene ngage Leaming.All Rights Reserved
Solution Reaction at positions B and b are identical so a total of only three products are formed Worked Example Predicting the Product of an Allylic Bromination Reaction

Preparing Alkyl Halides from Alcohols Many common methods have been developed to transform alcohols into alkyl halides Treat the alcohol with HCI,HBr,or HI Simplest method The reaction works best with tertiary alcohols,RaCOH Primary and secondary alcohols react slowly and at higher reaction temperatures + H20 HH R H OH R -OH R OH R OH Methyl Primary Secondary Tertiary Reactivity
Many common methods have been developed to transform alcohols into alkyl halides ▪ Treat the alcohol with HCl, HBr, or HI ▪ Simplest method ▪ The reaction works best with tertiary alcohols, R3COH ▪ Primary and secondary alcohols react slowly and at higher reaction temperatures Preparing Alkyl Halides from Alcohols