
Kinds of Organic Reactions Rearrangement reactions Occur when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product Rearrangement of dihydroxyacetone phosphate into its constitutional isomer glyceraldehyde 3-phosphate (a step in the metabolism of carbohydrates) 0 OH This reactant...2-03P C、 ,0H 2-030 ...gives this isomeric product H HH H H H Dihydroxyacetone Glyceraldehyde phosphate 3-phosphate Cngage Leaming All Rights Reserved
Rearrangement reactions ▪ Occur when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product ▪ Rearrangement of dihydroxyacetone phosphate into its constitutional isomer glyceraldehyde 3-phosphate (a step in the metabolism of carbohydrates) Kinds of Organic Reactions
6-2 How Organic Reactions Occur: Mechanisms Reaction Mechanism An overall description of how a reaction occurs at each stage of a chemical transformation Which bonds are broken and in what order Which bonds are formed and in what order -What is the relative rate of each step A complete mechanism accounts for all reactants consumed and all products formed
Reaction Mechanism ▪ An overall description of how a reaction occurs at each stage of a chemical transformation ▪ Which bonds are broken and in what order ▪ Which bonds are formed and in what order ▪ What is the relative rate of each step ▪ A complete mechanism accounts for all reactants consumed and all products formed 6-2 How Organic Reactions Occur: Mechanisms
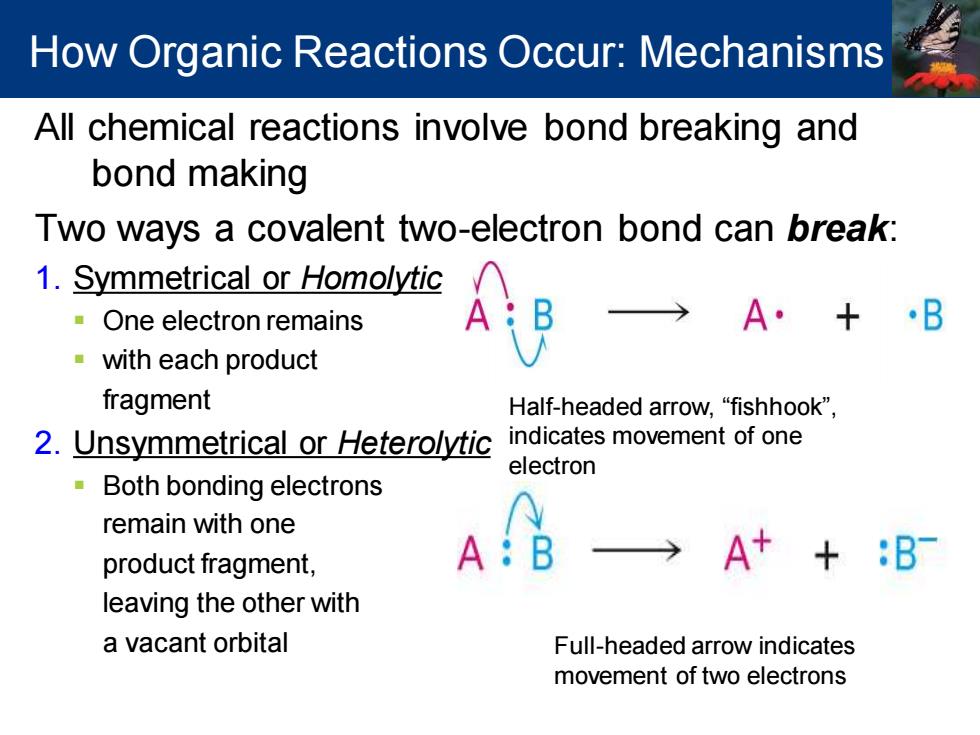
How Organic Reactions Occur:Mechanisms All chemical reactions involve bond breaking and bond making Two ways a covalent two-electron bond can break: 1.Symmetrical or Homolytic One electron remains A·+·B with each product fragment Half-headed arrow,"fishhook", 2.Unsymmetrical or Heterolytic indicates movement of one electron 。 Both bonding electrons remain with one product fragment, A A++:B leaving the other with a vacant orbital Full-headed arrow indicates movement of two electrons
All chemical reactions involve bond breaking and bond making Two ways a covalent two-electron bond can break: 1. Symmetrical or Homolytic ▪ One electron remains ▪ with each product fragment 2. Unsymmetrical or Heterolytic ▪ Both bonding electrons remain with one product fragment, leaving the other with a vacant orbital Half-headed arrow, “fishhook”, indicates movement of one electron Full-headed arrow indicates movement of two electrons How Organic Reactions Occur: Mechanisms
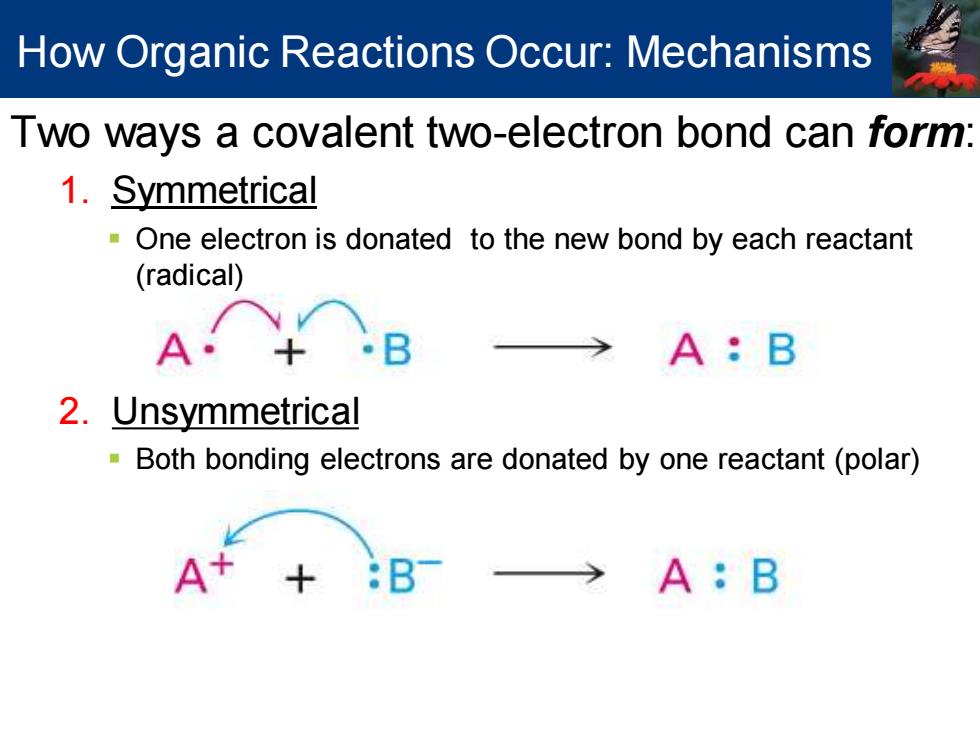
How Organic Reactions Occur:Mechanisms Two ways a covalent two-electron bond can form: 1.Symmetrical -One electron is donated to the new bond by each reactant (radical) A· A:B 2.Unsymmetrical Both bonding electrons are donated by one reactant(polar) A A:B
Two ways a covalent two-electron bond can form: 1. Symmetrical ▪ One electron is donated to the new bond by each reactant (radical) 2. Unsymmetrical ▪ Both bonding electrons are donated by one reactant (polar) How Organic Reactions Occur: Mechanisms
How Organic Reactions Occur:Mechanisms Radical reaction Process that involves symmetrical bond breaking and bond making Radical (free radical) A neutral chemical species that contains an odd number of electrons and has a single,unpaired electron in one of its orbitals Polar reaction Process that involves unsymmetrica/bond breaking and bond making Involve species that have an even number of electrons (have only electron pairs in their orbitals) Common in both organic and biological chemistry
Radical reaction ▪ Process that involves symmetrical bond breaking and bond making ▪ Radical (free radical) ▪ A neutral chemical species that contains an odd number of electrons and has a single, unpaired electron in one of its orbitals Polar reaction ▪ Process that involves unsymmetrical bond breaking and bond making ▪ Involve species that have an even number of electrons (have only electron pairs in their orbitals) ▪ Common in both organic and biological chemistry How Organic Reactions Occur: Mechanisms