
SOLVAY CONFERENCE 1927 colourized by pastincolour.com A.PICARD E.HENRIOT P.EHRENFEST Ed.HERSEN Th.DE DONDER E.SCHRODINGER E.VERSCHAFFELT W.PAUL W.HEISENBERG R.H FOWLER L.BRILLOUIN P.DEBYE M.KNUDSEN W.L.BRAGG H.A.KRAMERS P.A.M.DIRAC A.H.COMPTON L,de BROGLE M.BORN N.BOHR I.LANGMUIR M.PLANCK Mme CURIE H.A.LORENTZ A.EINSTEIN P.LANGEVIN Ch.E.GUYE C.T.R.WILSON O.W.RICHARDSON Absents:Sir W.H.BRAGG.H.DESLANDRES et E.VAN AUBEL
经典物理学剩 下的两朵乌云 量子力学 的发展
Schrodinger's equation in quantum mechanics Schrodinger equation as Newton's law in classical mechanics Schrodinger part of a monument in front (wave)Equation atoms,molecules,and subatomic particles of Warsaw University's free,bound,or localized Centre of New Technologies The linear partial differential equation describes the wave-function changes over time of a physical system in which quantum effects,such as wave-particle duality,are significant -气阿6可 The Nobel Prize in Physics defines the state of the system at each spatial 1933 position,and time(also called a "state function" dA:BA
Schrödinger (wave) Equation The Nobel Prize in Physics 1933 Schrödinger equation as part of a monument in front of Warsaw University's Centre of New Technologies Schrödinger's equation in quantum mechanics = Newton's law in classical mechanics atoms, molecules, and subatomic particles free, bound, or localized The linear partial differential equation describes the wave-function changes over time of a physical system in which quantum effects, such as wave–particle duality, are significant. defines the state of the system at each spatial position, and time (also called a "state function")

Schrodinger (wave)Equation iis the imaginary unit; Time-dependent Schrodinger equation(general) h=h/2nt is the reduced Planck constant; 2 h证更()=(④》 d/dt is a derivative with respect to time t; (psi)is the state vector of the quantum system; The position-space wave function of the quantum system rand t are the position vector and time; is the state vector in terms of the position eigenvector Ir), s,均=〉 Ais the Hamiltonian operator which The momentum-space wave function p,t)=〉 characterizes the total energy of the system under consideration. Time-dependent Schrodinger equation in position basis u is the particle's"reduced mass"; (single nonrelativistic particle) V is its potential energy; h 亚(r,t)= V2 is the Laplacian (a Differential Operator); 8t 2μ 亚(r,t) is the position-space wave function. Total energy Kinetic energy Potential energy
i is the imaginary unit; ħ= h/2π is the reduced Planck constant; d/dt is a derivative with respect to time t; Ψ (psi) is the state vector of the quantum system; r and t are the position vector and time; Ĥ is the Hamiltonian operator which characterizes the total energy of the system under consideration. μ is the particle‘s “reduced mass”; V is its potential energy; ∇ 2 is the Laplacian (a Differential Operator); Ψ is the position-space wave function. Total energy = Kinetic energy + Potential energy The position-space wave function of the quantum system is the state vector in terms of the position eigenvector |r⟩, Schrödinger (wave) Equation The momentum-space wave function
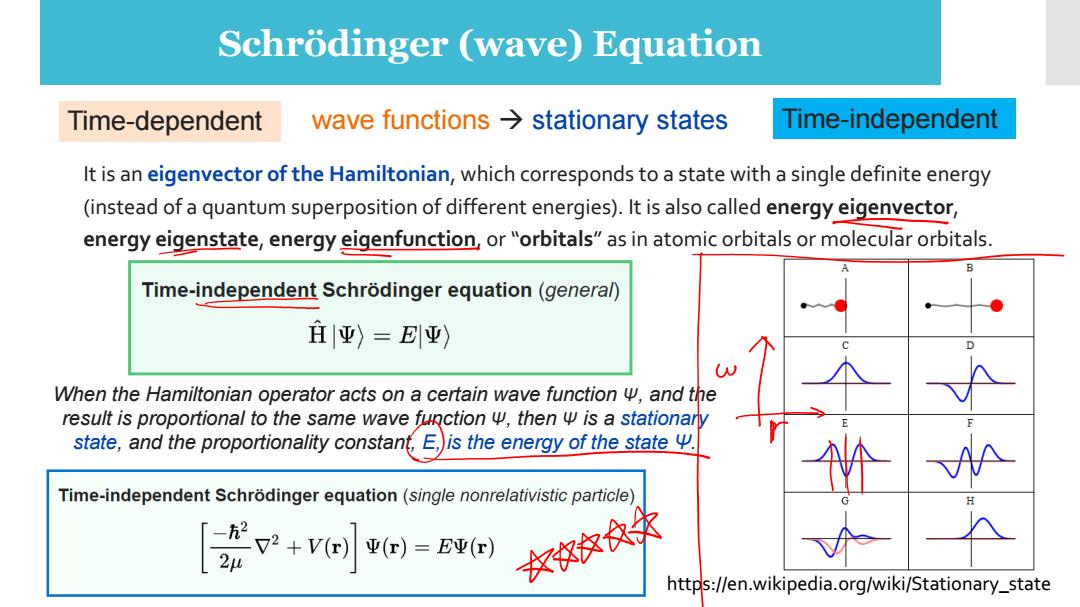
Schrodinger (wave)Equation Time-dependent wave functions>stationary states Time-independent It is an eigenvector of the Hamiltonian,which corresponds to a state with a single definite energy (instead of a quantum superposition of different energies).It is also called energy eigenvector, energy eigenstate,energy eigenfunction,or"orbitals"as in atomic orbitals or molecular orbitals. Time-independent Schrodinger equation(general) 庄|亚)=E平) When the Hamiltonian operator acts on a certain wave function w,and the result is proportional to the same wave function w,then w is a stationary state,and the proportionality constant,E,)is the energy of the state y. Time-independent Schrodinger equation(single nonrelativistic particle) [p2+v小=5 )牧效来 https://en.wikipedia.org/wiki/Stationary_state
Schrödinger (wave) Equation wave functions → stationary states It is an eigenvector of the Hamiltonian, which corresponds to a state with a single definite energy (instead of a quantum superposition of different energies). It is also called energy eigenvector, energy eigenstate, energy eigenfunction, or “orbitals” as in atomic orbitals or molecular orbitals. Time-dependent Time-independent When the Hamiltonian operator acts on a certain wave function Ψ, and the result is proportional to the same wave function Ψ, then Ψ is a stationary state, and the proportionality constant, E, is the energy of the state Ψ. https://en.wikipedia.org/wiki/Stationary_state

Schrodinger (wave)Equation TAUSEN口 [p+g-w 1▣a回 SCHILLING 1000 YG,2,,rw)=EΨ'(,2,rw) external potential e--e-interaction ·平cannot be found analytically complete "numerical"solution is possible but inefficient ·Complexity: basis set sizeNumber of electrons Hsrd problem tosve boQ@ve队 many electron system Schrodinger equation
Schrödinger (wave) Equation • Y cannot be found analytically • complete “numerical” solution is possible but inefficient • Complexity: basis set sizeNumber of electrons Hard problem to solve Schrödinger equation