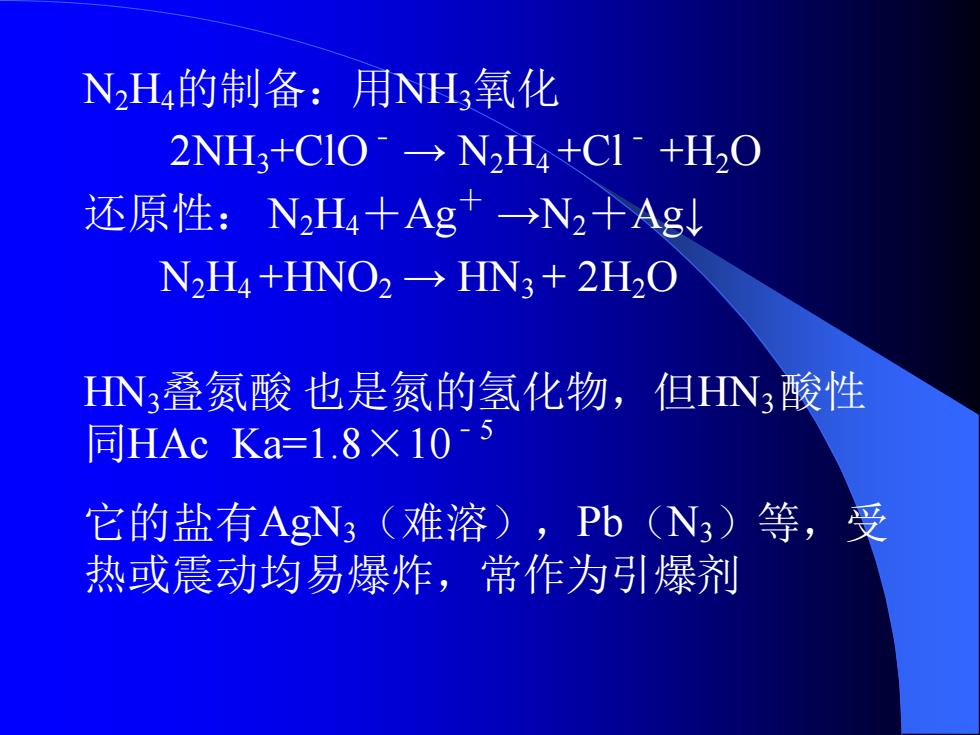
N2H的制备:用NH3氧化 2NH3+CIO°→N2H4+C1+H2O 还原性:N2H4十Ag→N2十Ag N2H4+HNO2→HN3+2H2O HN3叠氮酸也是氮的氢化物,但HN3酸性 同HAc Ka=1.8×105 它的盐有AgN(难溶),Pb(N3)等,受 热或震动均易爆炸,常作为引爆剂
N2H4的制备:用NH3氧化 2NH3+ClO﹣ → N2H4 +Cl﹣ +H2O 还原性: N2H4+Ag+ →N2+Ag↓ N2H4 +HNO2 → HN3 + 2H2O HN3叠氮酸 也是氮的氢化物,但HN3 酸性 同HAc Ka=1.8×10﹣5 它的盐有AgN3(难溶),Pb(N3)等,受 热或震动均易爆炸,常作为引爆剂

。NH,OH:较强还原剂(不引起污染) NH2OH+AgBr→Ag+HBr+H2O+N2(或N2O) N2十5H+十4e=N2H5pA°=-0.23V N2+2H++2H2O+2e=2NH2OH0a°三-1.87V N2+4H0+4e=N2H4+4OHpB°=-1.51V N2+4H20+2e =2NH2OH+20HT 0B°=-3.04V
⚫ NH2OH:较强还原剂(不引起污染) NH2OH+AgBr→Ag+HBr+H2O+N2 (或N2O) N2+5H + +4e =N2H5 + A =-0.23V N2+2H + +2H2O+2e =2NH2OH A =-1.87V N2+4H2O +4e =N2H4 +4OH- B =-1.51V N2+4H2O+2e =2NH2OH+2OH- B =-3.04V