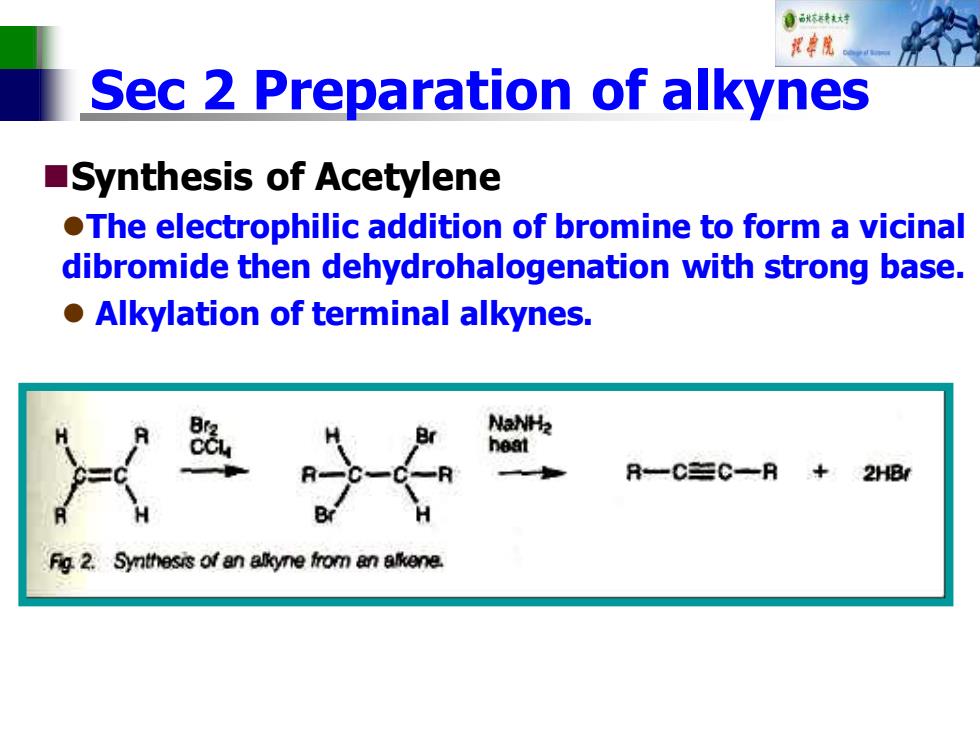
自秋精达对 视中院 Sec 2 Preparation of alkynes Synthesis of Acetylene .The electrophilic addition of bromine to form a vicinal dibromide then dehydrohalogenation with strong base. o Alkylation of terminal alkynes. NaNH2 heat R-C=C一R+2HB/ Fig 2.Synthesis of an allkyne from an alkene
Sec 2 Preparation of alkynes ◼Synthesis of Acetylene ⚫The electrophilic addition of bromine to form a vicinal dibromide then dehydrohalogenation with strong base. ⚫ Alkylation of terminal alkynes
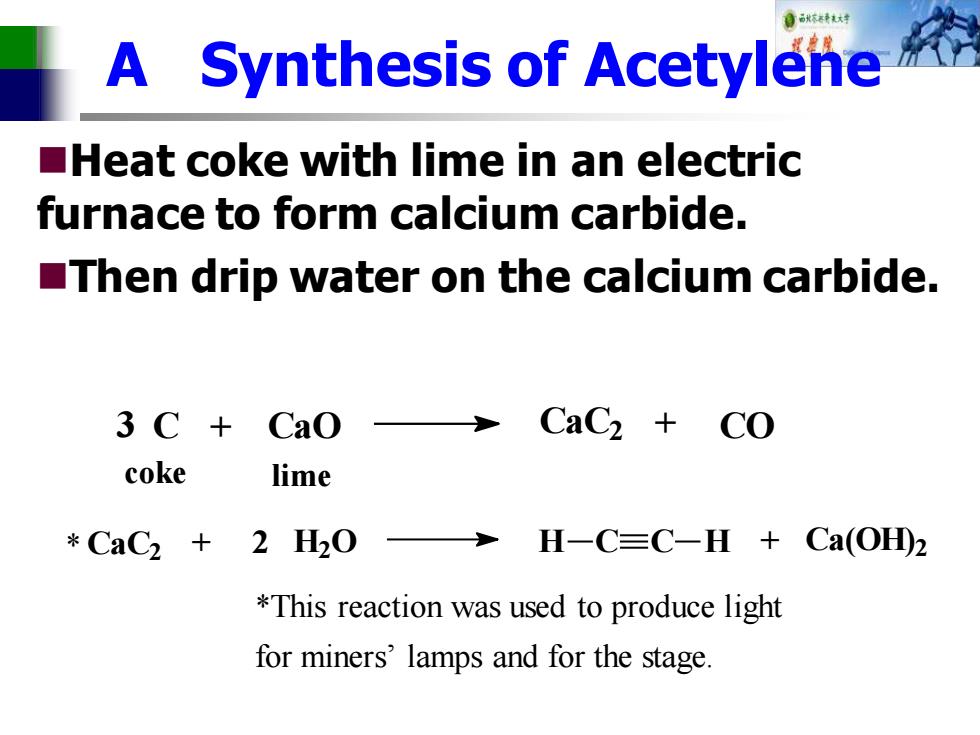
目秋不还转材对 A Synthesis of Acetylene Heat coke with lime in an electric furnace to form calcium carbide. Then drip water on the calcium carbide. 3C+ Cao CaCz coke lime *CaC2+2H20 H-C=C-H Ca(OH)2 *This reaction was used to produce light for miners'lamps and for the stage
A Synthesis of Acetylene ◼Heat coke with lime in an electric furnace to form calcium carbide. ◼Then drip water on the calcium carbide. CaC2 + 2 H2O H C C H + Ca(OH) 2 3 C + CaO CaC2 + CO coke lime *This reaction was used to produce light for miners’ lamps and for the stage. *
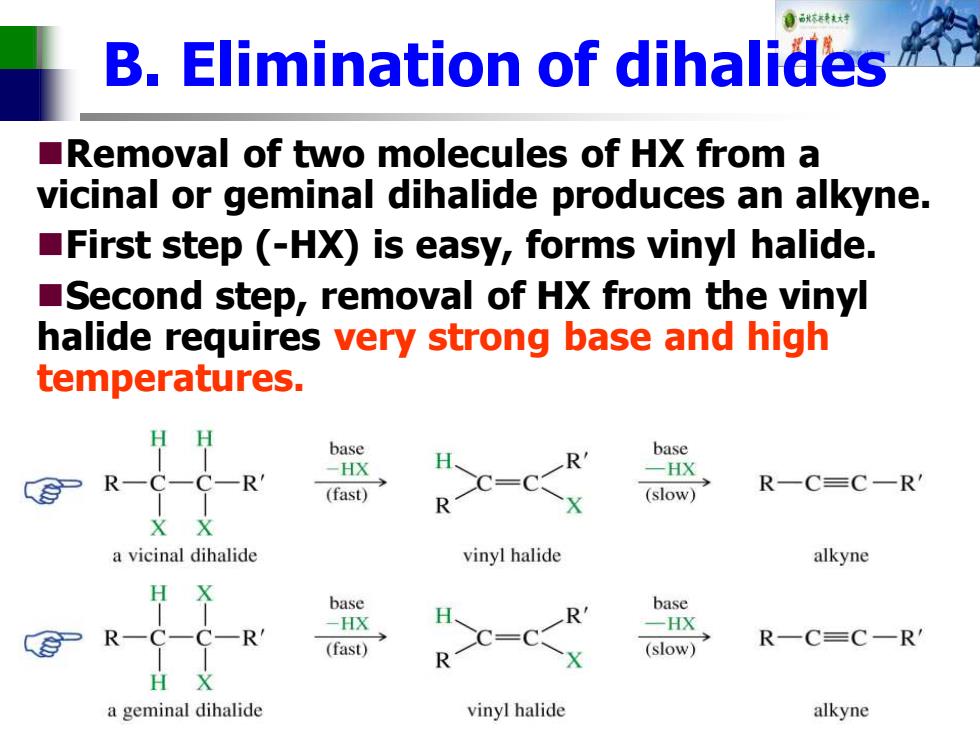
自秋精达对 B.Elimination of dihalides Removal of two molecules of HX from a vicinal or geminal dihalide produces an alkyne. First step (-HX)is easy,forms vinyl halide. Second step,removal of HX from the vinyl halide requires very strong base and high temperatures. base base 一HX R- -HX (fast) R一C=C一R (slow) a vicinal dihalide vinyl halide alkyne H X base base -HX R -HX R-C- -C一R =0 (fast) (slow) R一C=C一R H X a geminal dihalide vinyl halide alkyne
B. Elimination of dihalides ◼Removal of two molecules of HX from a vicinal or geminal dihalide produces an alkyne. ◼First step (-HX) is easy, forms vinyl halide. ◼Second step, removal of HX from the vinyl halide requires very strong base and high temperatures
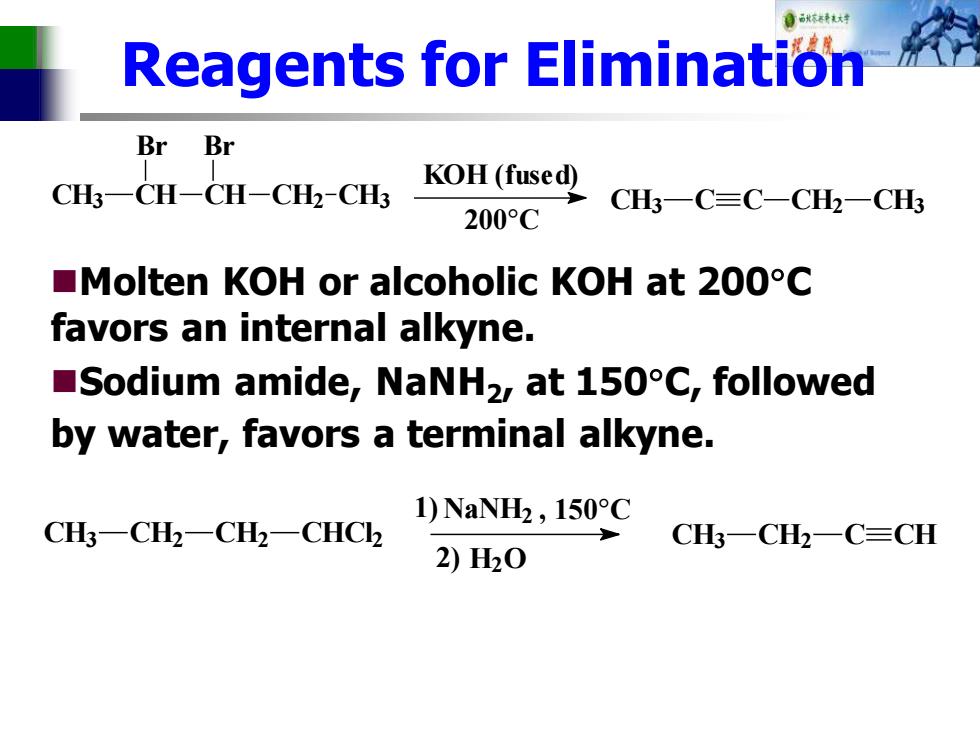
Reagents for Elimination Br Br CH3一CH-CH-CH2-CH KOH(fused) CH3一C≡C-CH2一CH3 200°C Molten KOH or alcoholic KOH at 200C favors an internal alkyne. Sodium amide,NaNHz,at 150C,followed by water,favors a terminal alkyne. 1)NaNH2,150°C CH一CH2一CH2一CHC2 CH一CH2一C=CH 2)H20
Reagents for Elimination ◼Molten KOH or alcoholic KOH at 200C favors an internal alkyne. ◼Sodium amide, NaNH2 , at 150C, followed by water, favors a terminal alkyne. CH3 C C CH2 CH3 200°C KOH (fused) CH3 CH CH CH2 CH3 Br Br , 150°C CH3 CH2 C CH 2) H2O 1) NaNH2 CH3 CH2 CH2 CHCl2