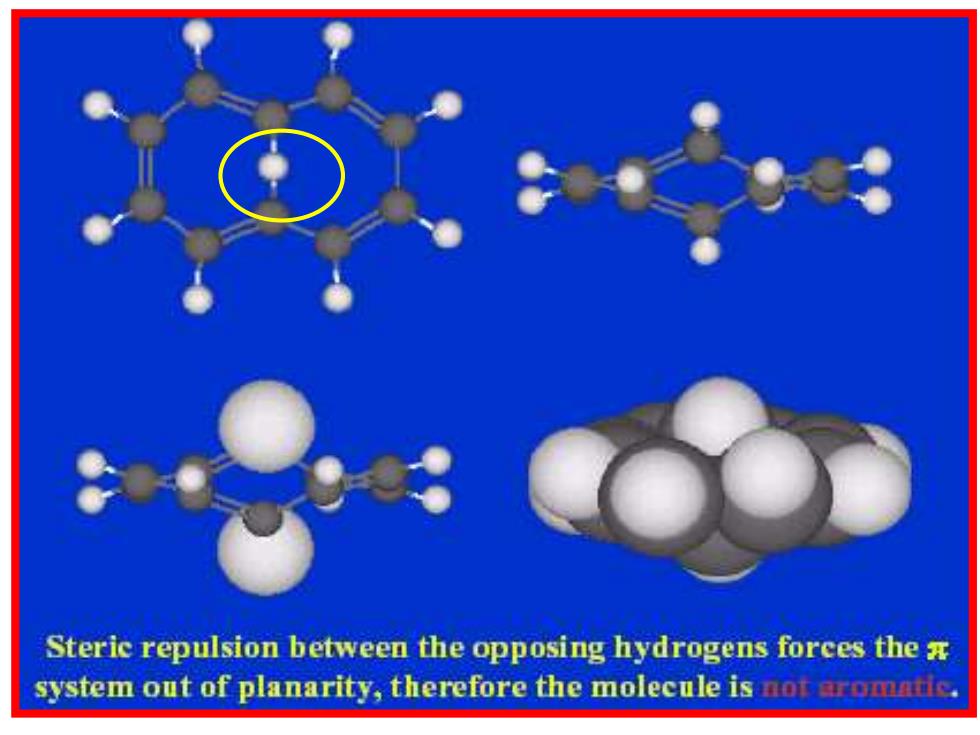
Steric repulsion between the opposing hydrogens forces the x system out of planarity,therefore the molecule is not aromatic
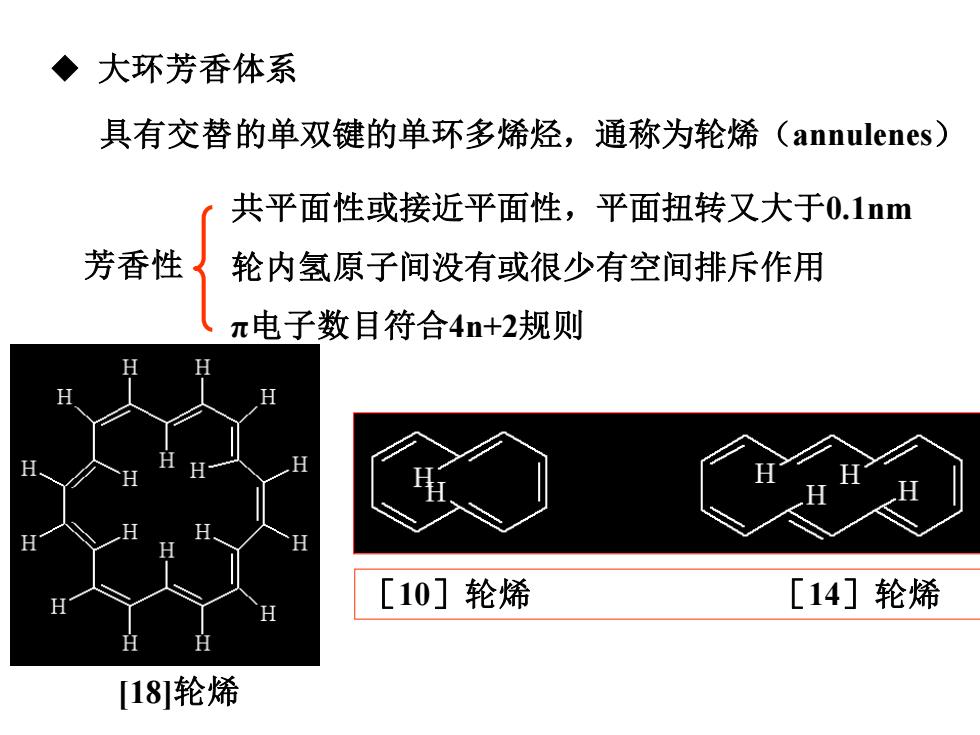
大环芳香体系 具有交替的单双键的单环多烯烃,通称为轮烯(annulenes) 共平面性或接近平面性,平面扭转又大于0.1nm 芳香性 轮内氢原子间没有或很少有空间排斥作用 π电子数目符合4n+2规则 H [10]轮烯 [14]轮烯 [18轮烯
◆ 大环芳香体系 具有交替的单双键的单环多烯烃,通称为轮烯(annulenes) 芳香性 共平面性或接近平面性,平面扭转又大于0.1nm 轮内氢原子间没有或很少有空间排斥作用 π电子数目符合4n+2规则 [18]轮烯 [10]轮烯 [14]轮烯
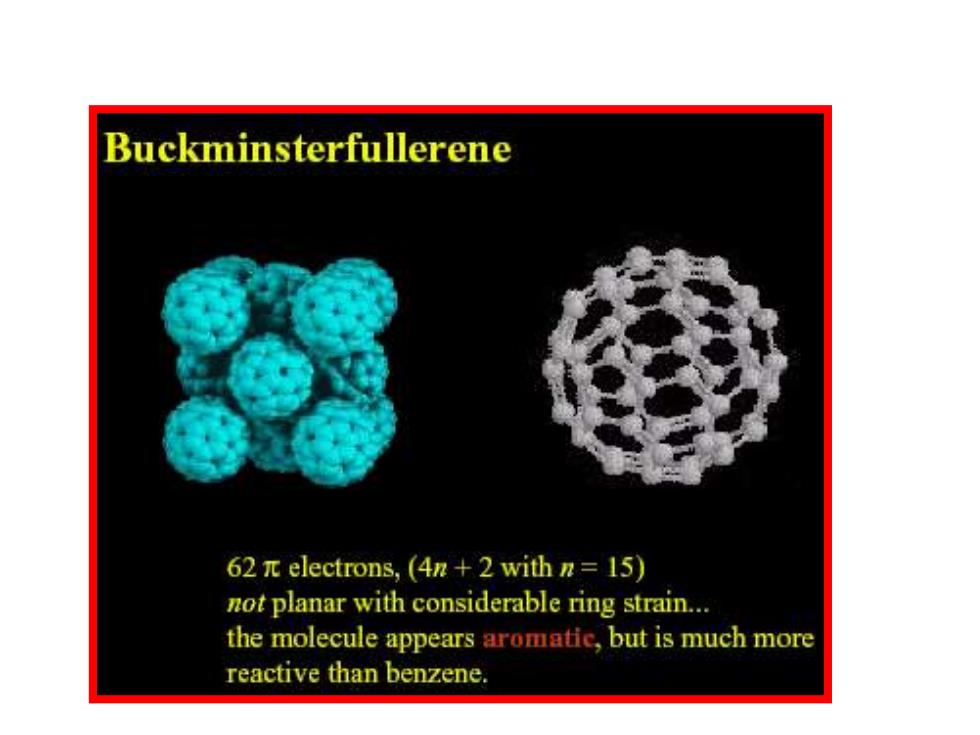
Buckminsterfullerene 62 electrons,(4n+2 with n=15) not planar with considerable ring strain... the molecule appears aromatic,but is much more reactive than benzene

are very rugged.They can survive collisions h metals and other materials at speeds in excess of 20,000 miles per hour,a speed that would tear most molecules apart 1mile=1.6 km 100m/s
1mile=1.6 km 100m/s
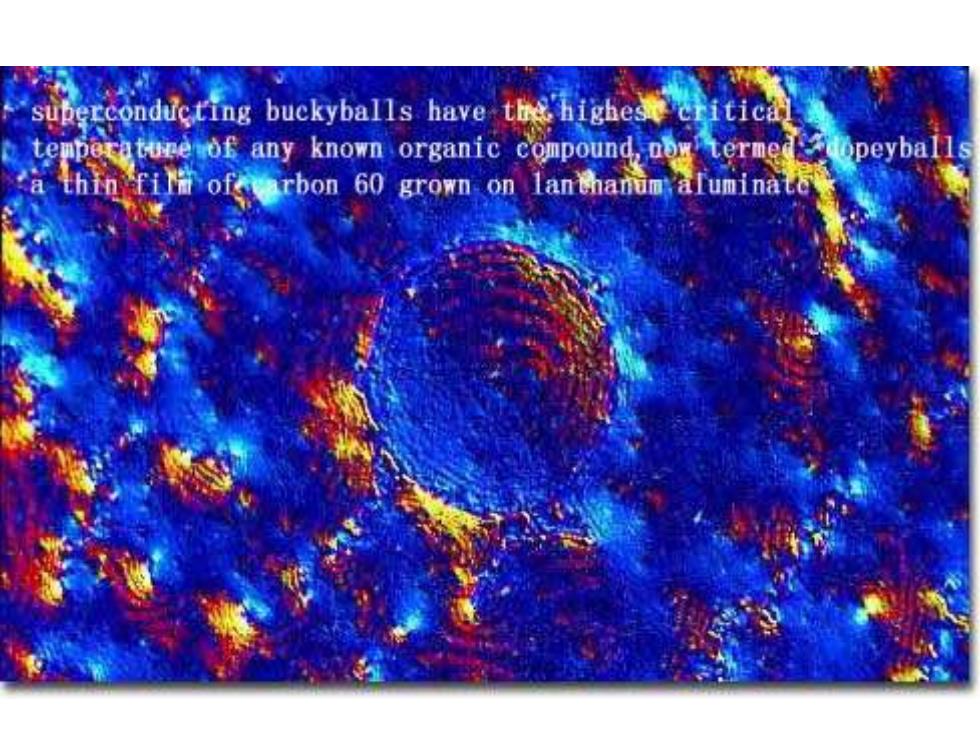
superconducting buckyballs have the highese critical ture of any known organic compound,now termed arbon 60 grown on