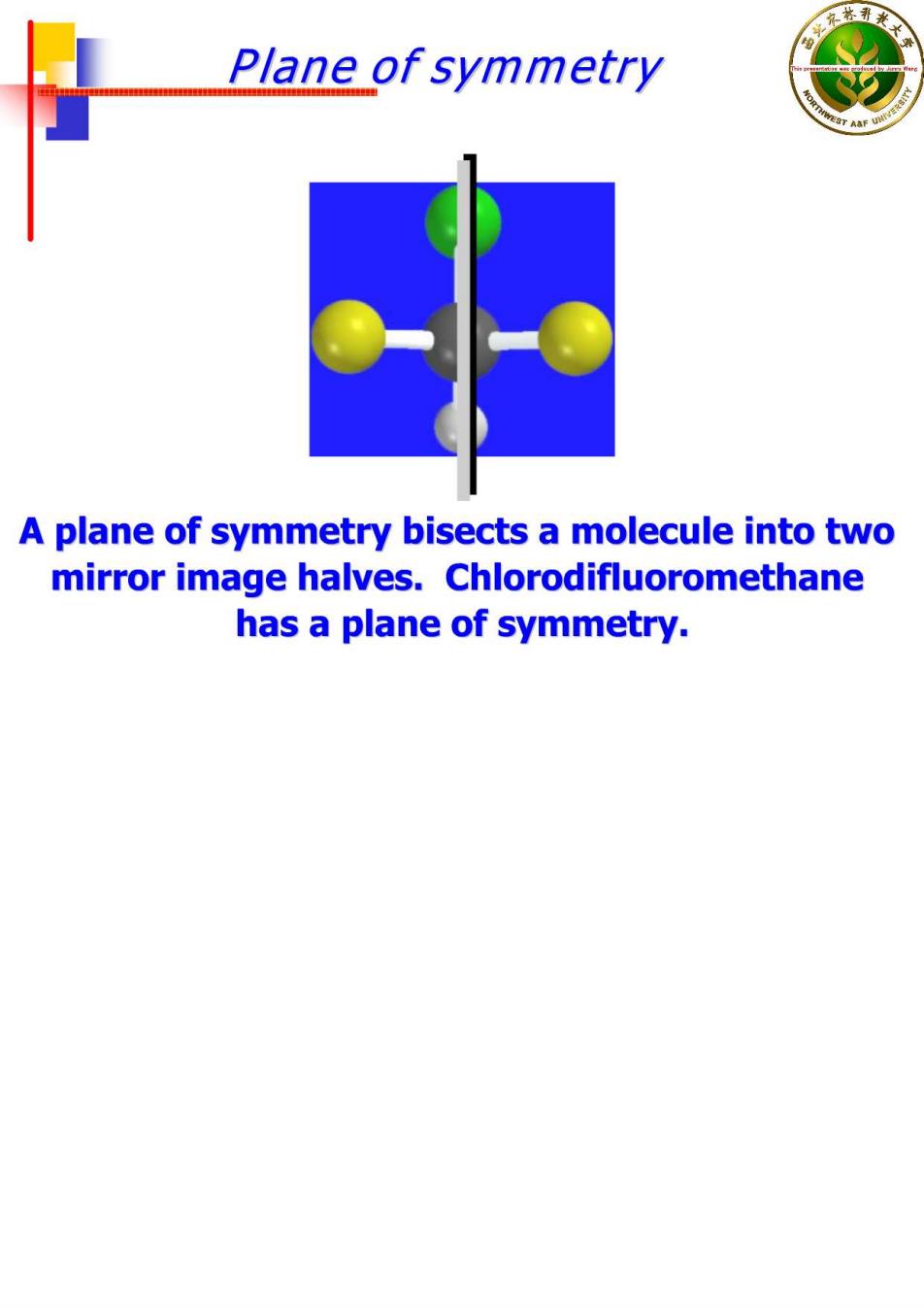
Plane of symmetry A plane of symmetry bisects a molecule into two mirror image halves.Chlorodifluoromethane has a plane of symmetry
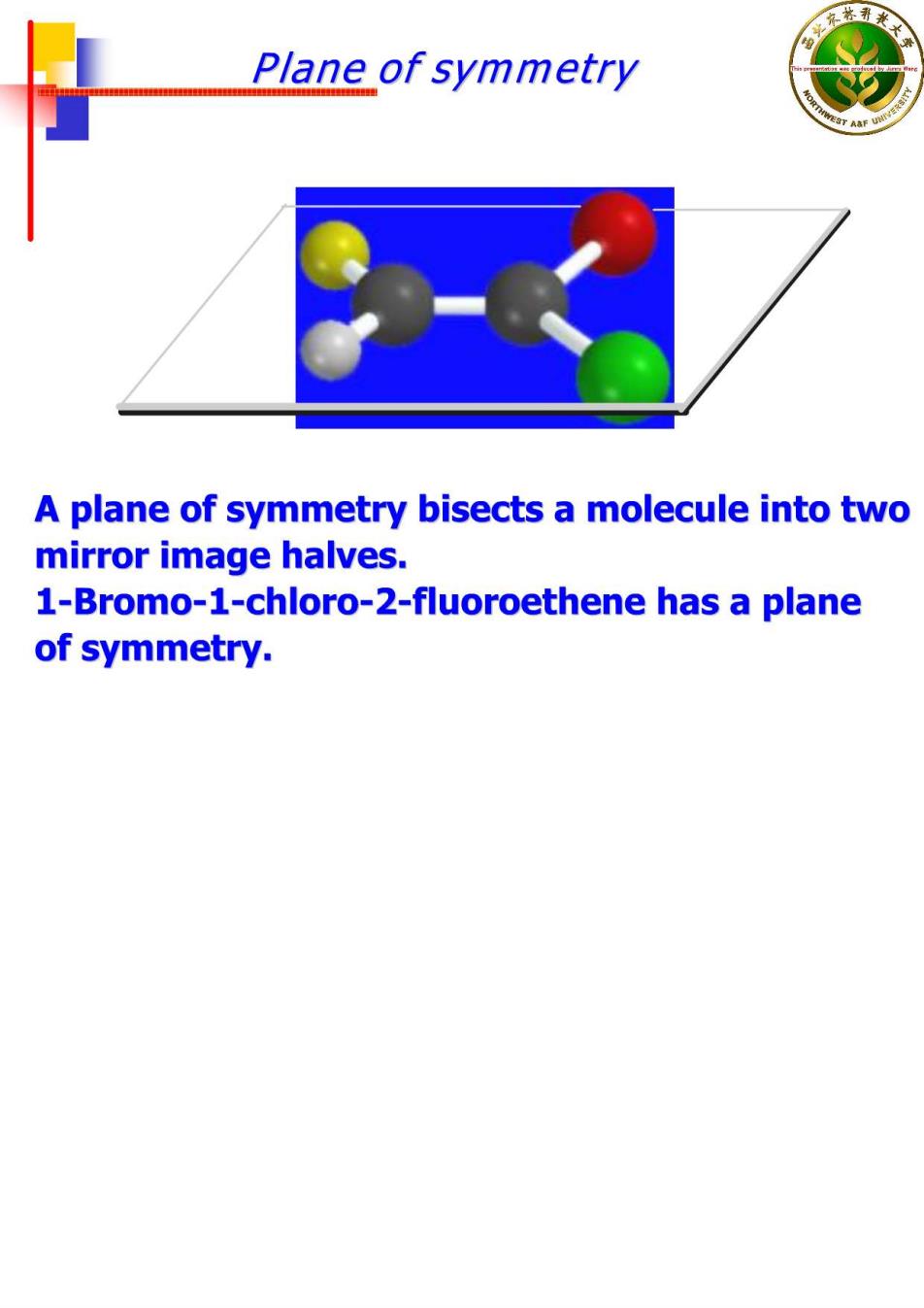
Plane of symmetry A plane of symmetry bisects a molecule into two mirror image halves. 1-Bromo-1-chloro-2-fluoroethene has a plane of symmetry
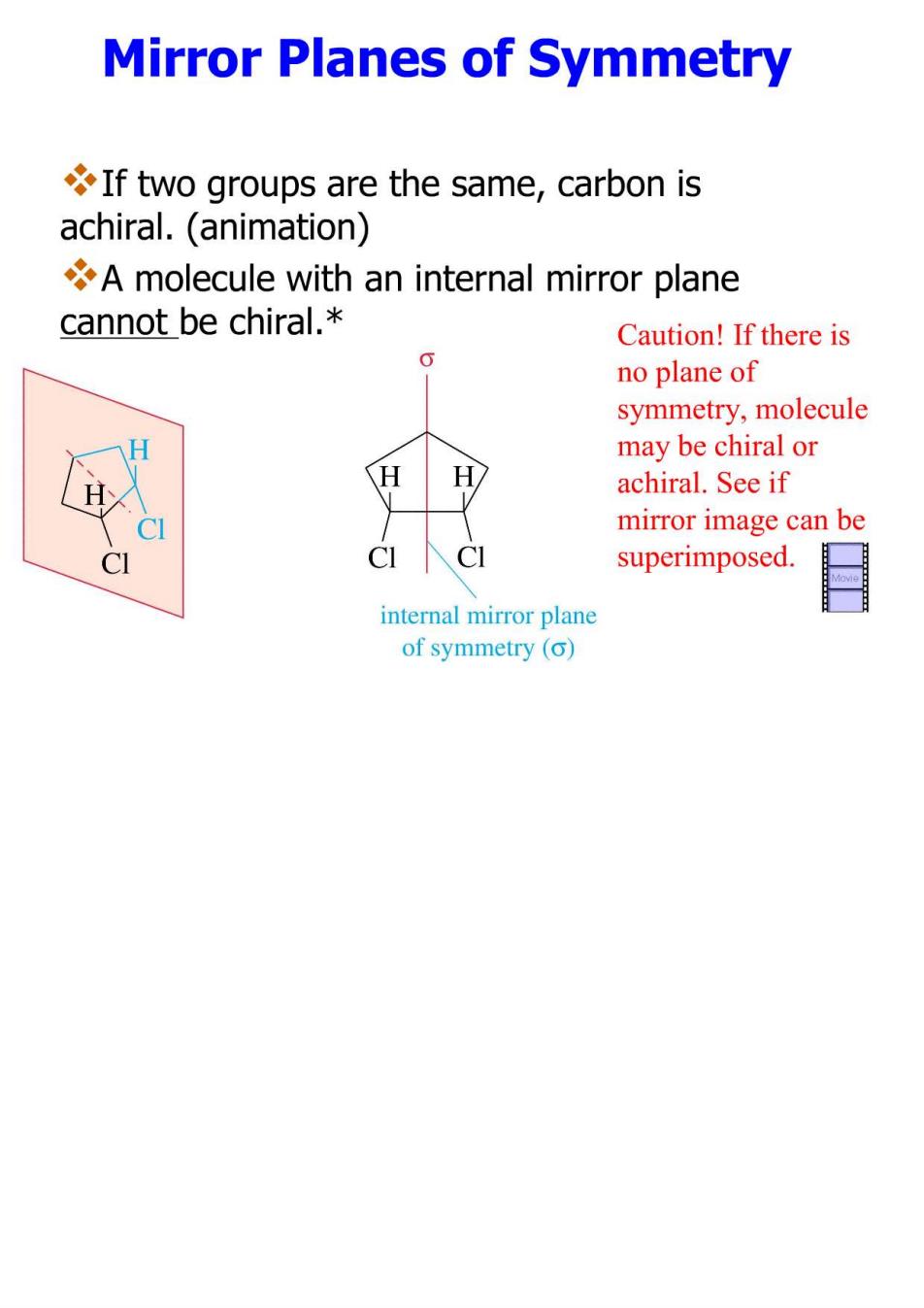
Mirror Planes of Symmetry If two groups are the same,carbon is achiral.(animation) A molecule with an internal mirror plane cannot be chiral.* Caution!If there is no plane of symmetry,molecule H may be chiral or H achiral.See if CI mirror image can be CI superimposed. internal mirror plane of symmetry (o)
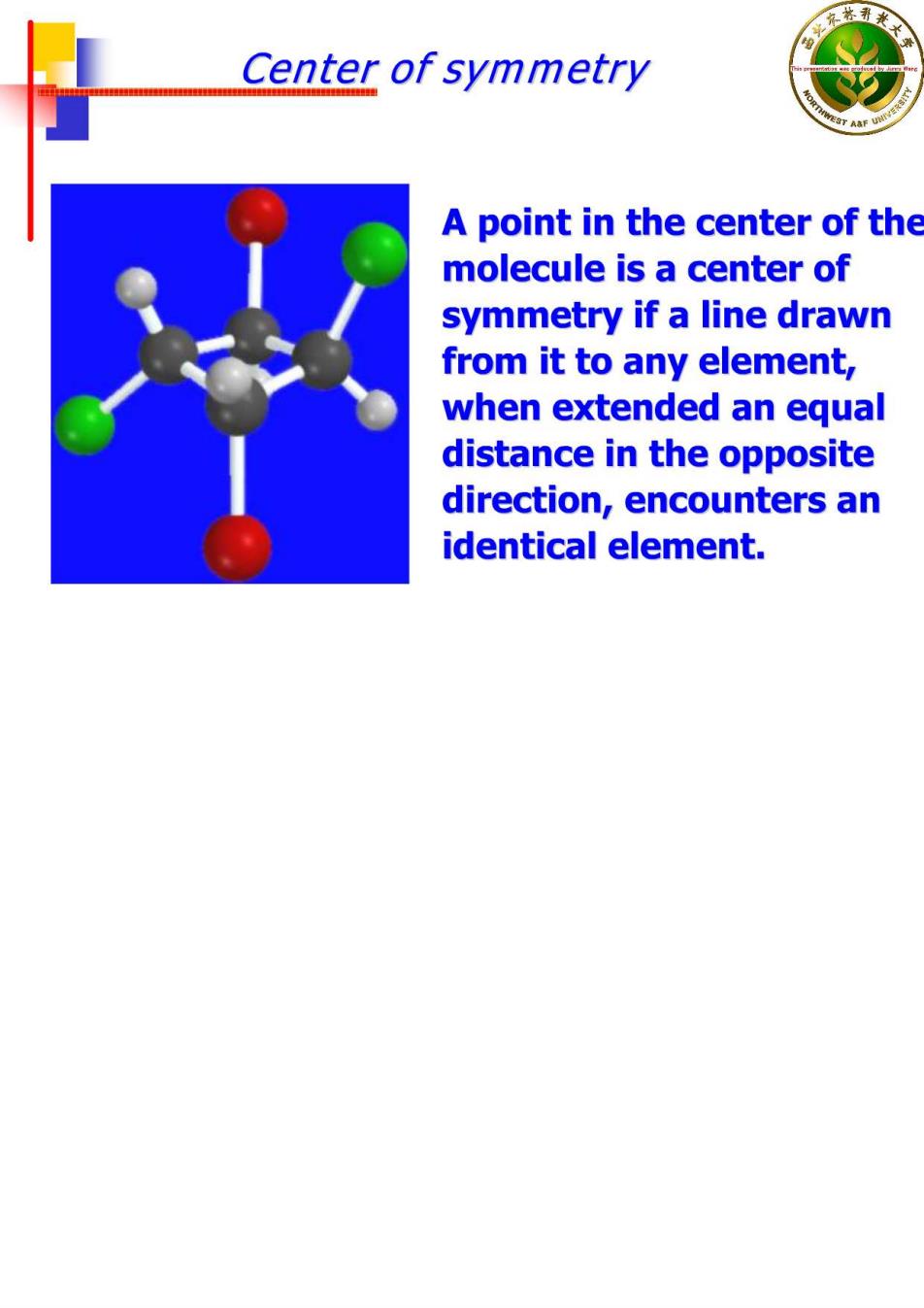
Center of symmetry A point in the center of the molecule is a center of symmetry if a line drawn from it to any element, when extended an equal distance in the opposite direction,encounters an identical element

手性与分子结构关系 冬手性、对称性与旋光性 ,有反映对称性的分子:物象重合,不具有旋光性,非手性分子 无对称性的分子:物象不重合,有旋光性,手性分子 必手性碳与手性分子二者关系 ,有手性碳的分子不一定就是手性分子 手性分子并不一定就含有手性碳 •只有一个手性碳的分子一定就是手性分子 必构型与旋光性 分子结构的不对称性导致,物象不重合;二者主要差异在于构 型不同; 必对称性、不对称性与手性: 对称因素:对称面(σ);对称中心() ,凡是没有反映对称性的分子称为手性分子 不对称性:找不到任何一个对称因素的分子;手性是不对称性 之一种