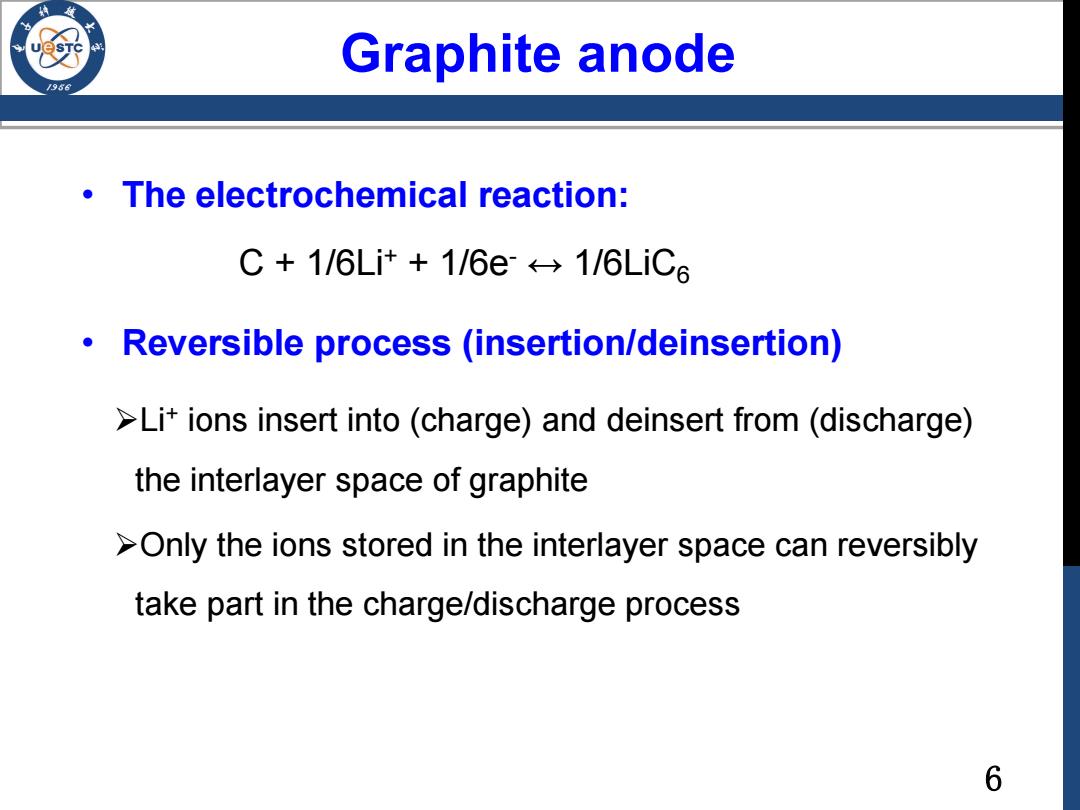
Graphite anode /98 The electrochemical reaction: C+1/6Lit+1/6e←→1/6LiC6 Reversible process (insertion/deinsertion) >Lit ions insert into(charge)and deinsert from (discharge) the interlayer space of graphite >Only the ions stored in the interlayer space can reversibly take part in the charge/discharge process 6
6 Graphite anode • The electrochemical reaction: C + 1/6Li+ + 1/6e- ↔ 1/6LiC6 • Reversible process (insertion/deinsertion) Li+ ions insert into (charge) and deinsert from (discharge) the interlayer space of graphite Only the ions stored in the interlayer space can reversibly take part in the charge/discharge process

Theoretical capacity of graphite /98 The electrochemical reaction: C+1/6Lit+1/6e←→1/6LiC6 >Molecular weight of C is 12 g/mol >So,the theoretical capacity is: 96500÷12÷3.6×1/6=372.3mAh/g 7
7 Theoretical capacity of graphite • The electrochemical reaction: C + 1/6Li+ + 1/6e- ↔ 1/6LiC6 Molecular weight of C is 12 g/mol So, the theoretical capacity is: 96500÷12÷3.6×1/6 = 372.3 mA·h/g