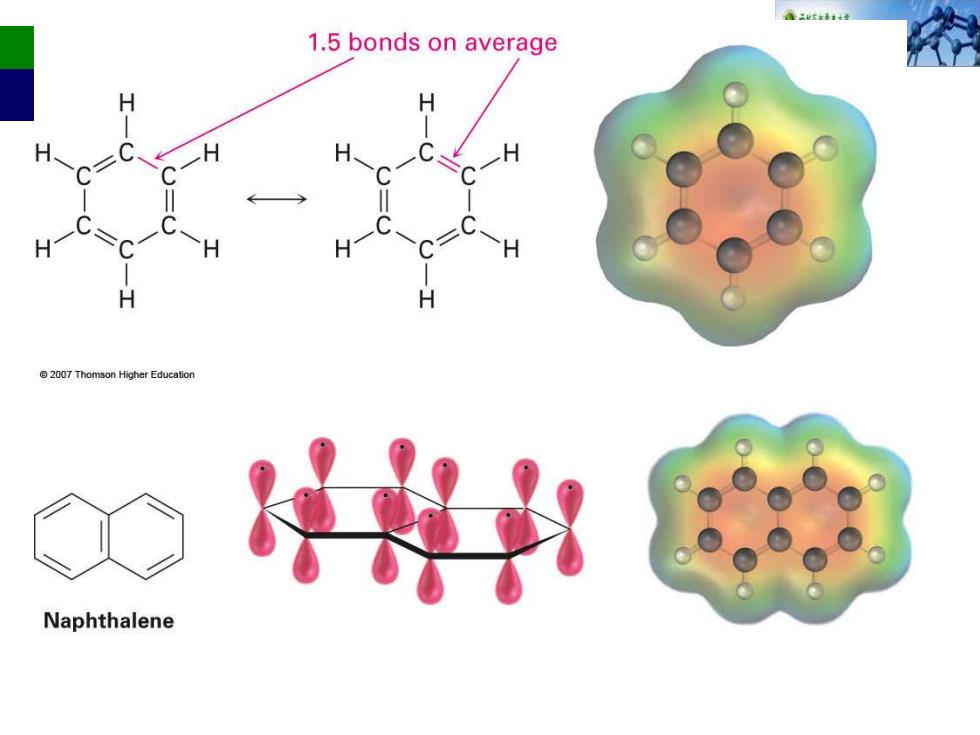
aaxi女 1.5 bonds on average H @2007 Thomson Higher Education Naphthalene
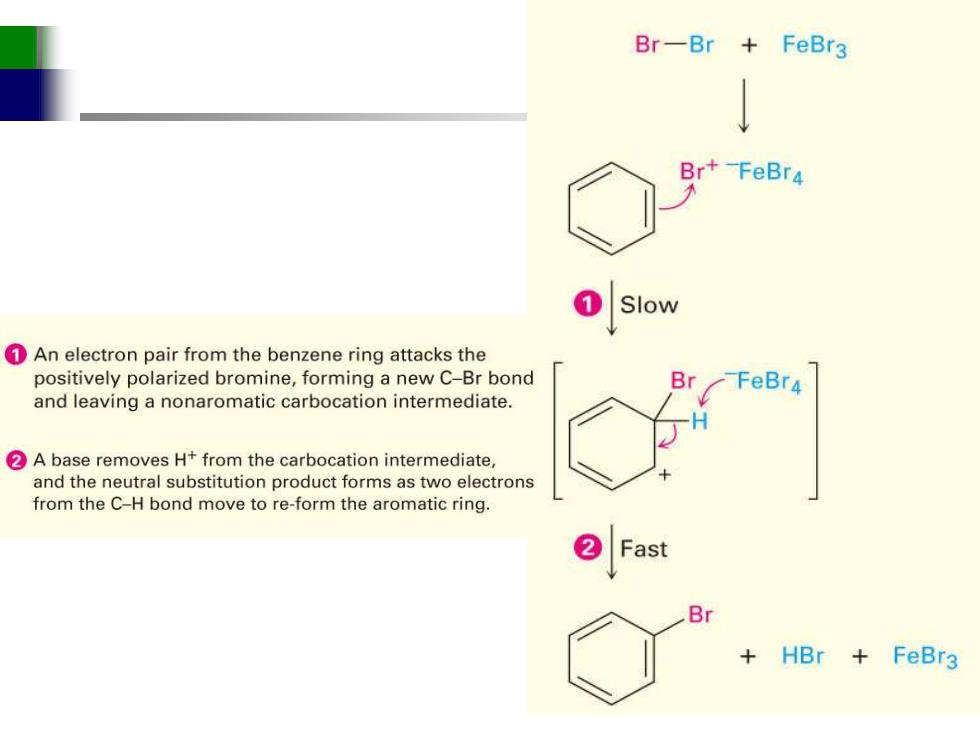
Br-Br+FeBr3 Br+-FeBr4 Slow 1An electron pair from the benzene ring attacks the positively polarized bromine,forming a new C-Br bond Br -FeBr4 and leaving a nonaromatic carbocation intermediate. 2A base removes H+from the carbocation intermediate, and the neutral substitution product forms as two electrons from the C-H bond move to re-form the aromatic ring. Fast Br +HBr+ FeBr3
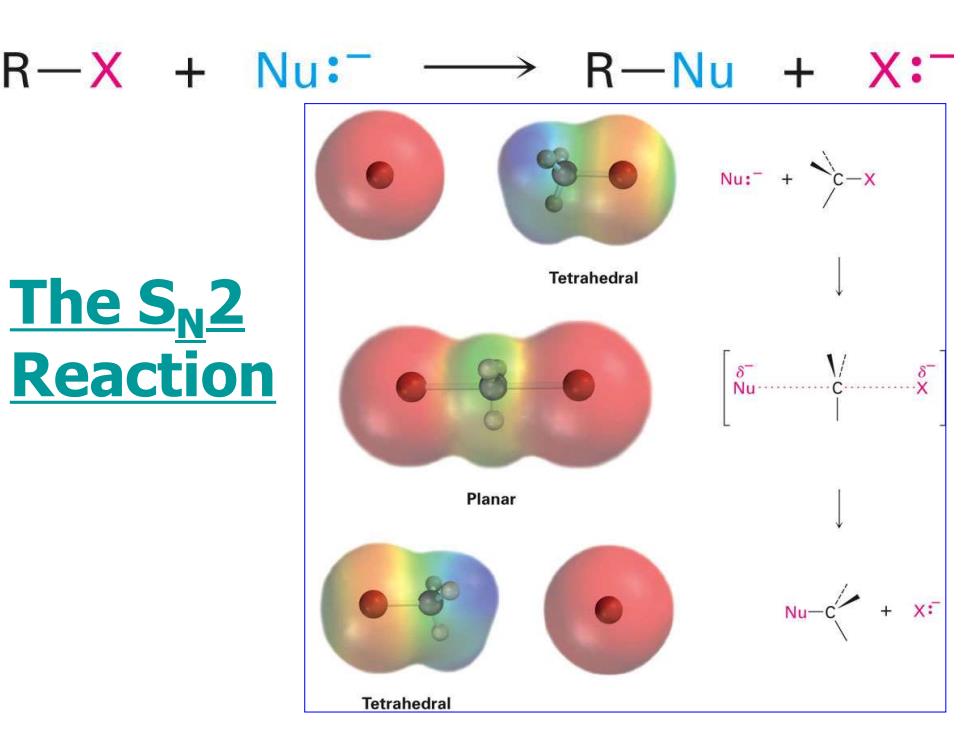
R一X+ Nu:-→ R一Nu+X:- Nu:-+ c-x Tetrahedral The SN2 Reaction Planar Nu-C X: Tetrahedral
The SN2 Reaction

自秋转大好 花串院 The E2 Reaction Base:.. X Anti periplanar reactant Anti transition state Alkene product
The E2 Reaction
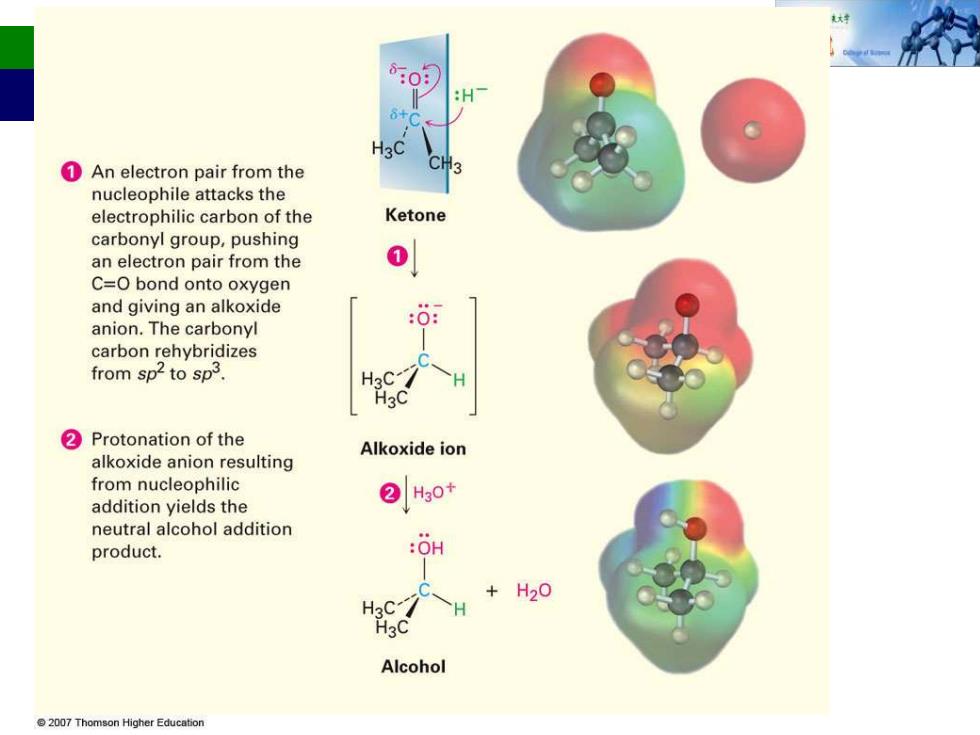
:H H3C 1An electron pair from the nucleophile attacks the electrophilic carbon of the Ketone carbonyl group,pushing an electron pair from the C=O bond onto oxygen and giving an alkoxide anion.The carbonyl carbon rehybridizes from sp2 to sp3. H3C ②Protonation of the alkoxide anion resulting Alkoxide ion from nucleophilic addition yields the ②Ho+ neutral alcohol addition product. :0H H20 H3C Alcohol 2007 Thomson Higher Education