
音秋标特大对 Sec 1 Classification Reactions can be classified as ■Acid/base reactions Functional group transformations; oone functional group can be converted into another. Normally these reactions are relatively straightforward and proceed in high yield carbon-carbon bond formations
Sec 1 Classification Reactions can be classified as ◼Acid/base reactions ◼Functional group transformations; ⚫one functional group can be converted into another. ⚫Normally these reactions are relatively straightforward and proceed in high yield ◼carbon-carbon bond formations
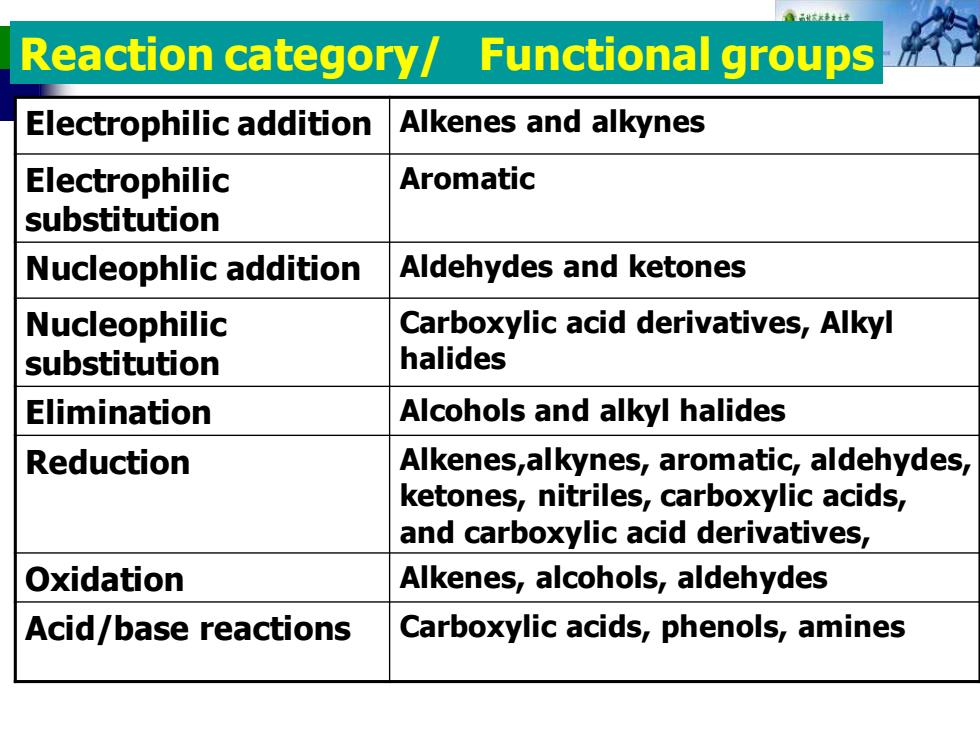
Reaction category/Functional groups Electrophilic addition Alkenes and alkynes Electrophilic Aromatic substitution Nucleophlic addition Aldehydes and ketones Nucleophilic Carboxylic acid derivatives,Alkyl substitution halides Elimination Alcohols and alkyl halides Reduction Alkenes,alkynes,aromatic,aldehydes, ketones,nitriles,carboxylic acids, and carboxylic acid derivatives, Oxidation Alkenes,alcohols,aldehydes Acid/base reactions Carboxylic acids,phenols,amines
Electrophilic addition Alkenes and alkynes Electrophilic substitution Aromatic Nucleophlic addition Aldehydes and ketones Nucleophilic substitution Carboxylic acid derivatives, Alkyl halides Elimination Alcohols and alkyl halides Reduction Alkenes,alkynes, aromatic, aldehydes, ketones, nitriles, carboxylic acids, and carboxylic acid derivatives, Oxidation Alkenes, alcohols, aldehydes Acid/base reactions Carboxylic acids, phenols, amines Reaction category/ Functional groups
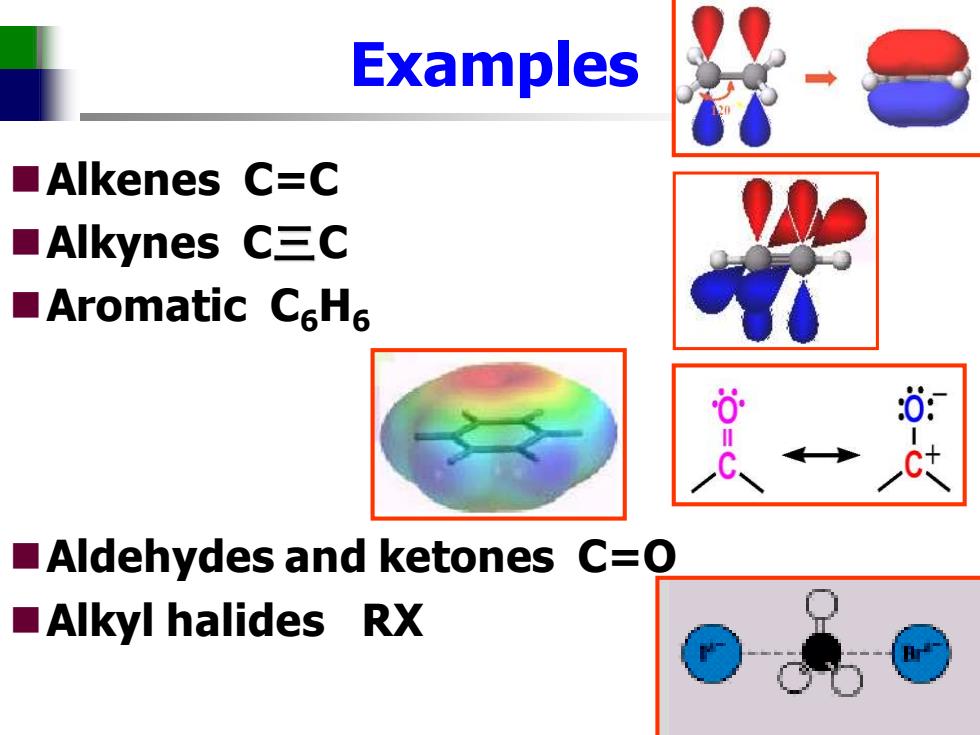
Examples ■Alkenes C=C ■Alkynes C三C ■Aromatic C6H6 0 0: Aldehydes and ketones C=0 ■Alkyl halides RX
Examples ◼Alkenes C=C ◼Alkynes C三C ◼Aromatic C6H6 ◼Aldehydes and ketones C=O ◼Alkyl halides RX
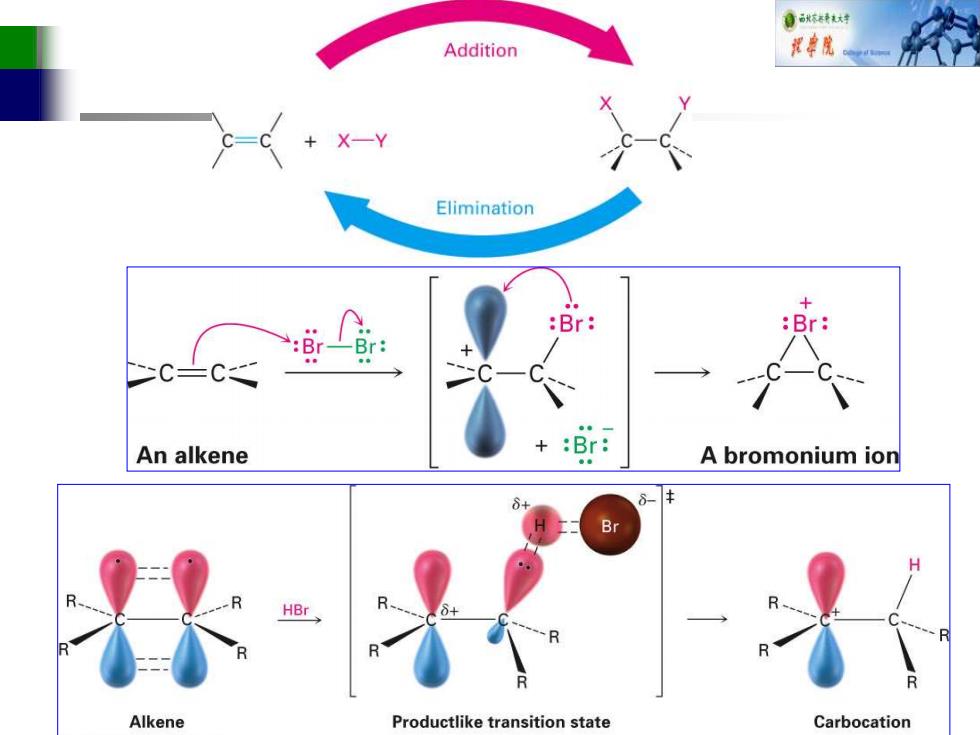
自秋转材对 Addition 视中院 X-Y Elimination + :Br :Br: :Br: Br: C-C An alkene +:Br: A bromonium ion 6- Br Alkene Productlike transition state Carbocation
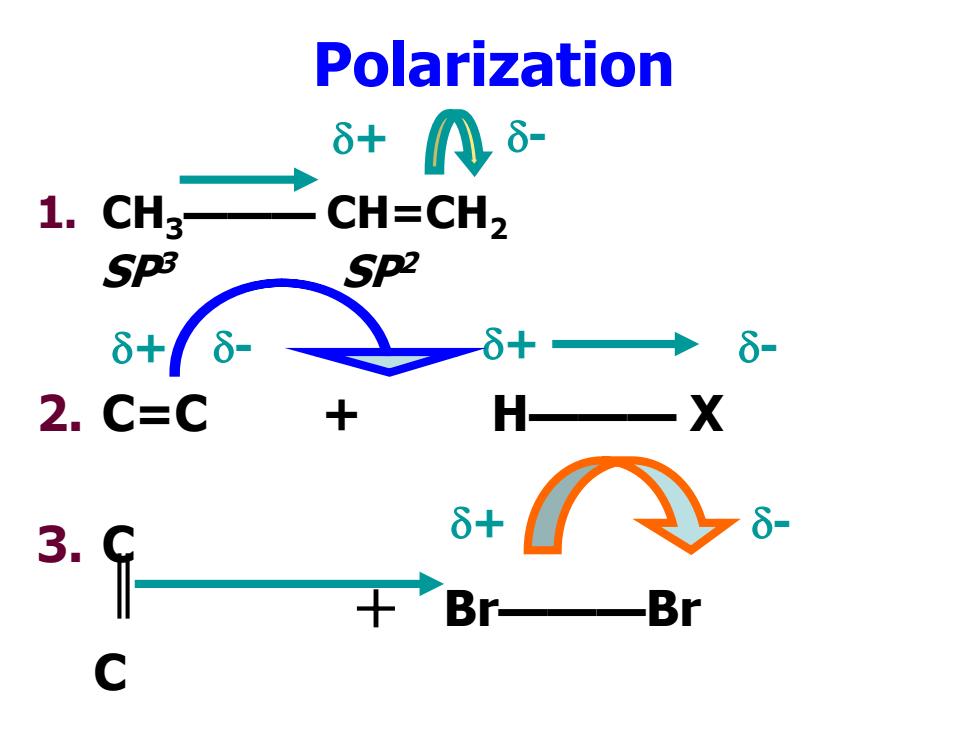
Polarization 6+八8- 1. CH3 CH=CH2 SpB Sp2 6+ 6- 6+ 6- 2.C=C H X 3. δ+ +Br Br
Polarization 1. CH3——— CH=CH2 SP3 SP2 2. C=C + H——— X 3. C + Br———Br C + - + - + - + -