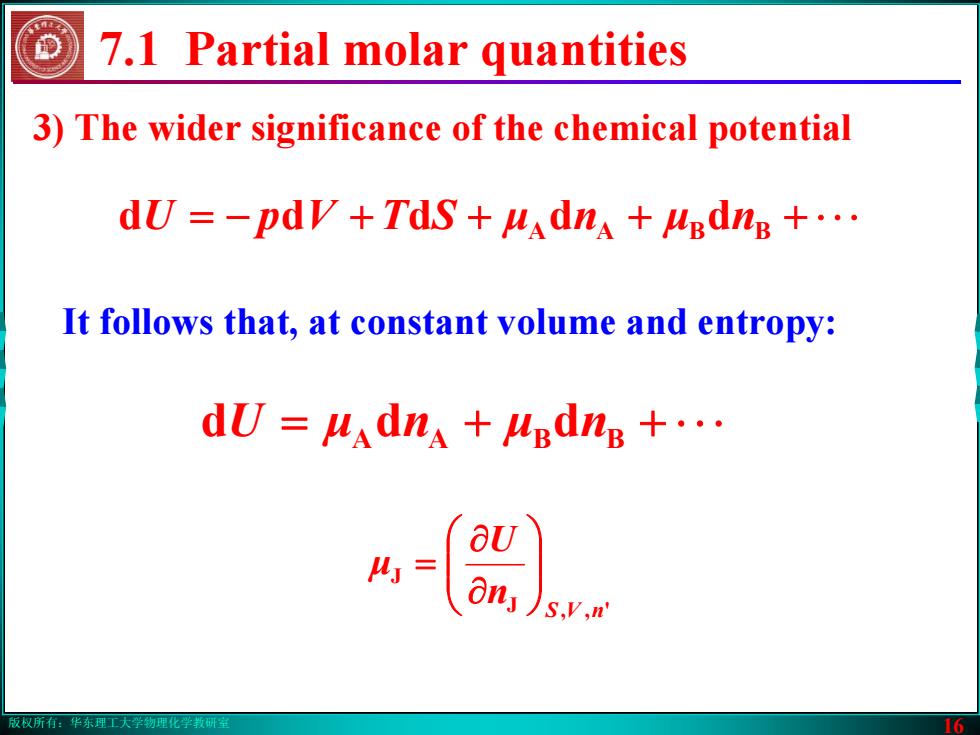
版权所有:华东理工大学物理化学教研室 16 3) The wider significance of the chemical potential 7.1 Partial molar quantities It follows that, at constant volume and entropy: −= + + + + ⋅ ⋅ ⋅ STVpU μ n μ ddddd nBBAA = + + ⋅ ⋅ ⋅ U μ n μ ddd nBBAA J ', J nVS n U μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ =
版权所有:华东理工大学物理化学教研室 16 3) The wider significance of the chemical potential 7.1 Partial molar quantities It follows that, at constant volume and entropy: −= + + + + ⋅ ⋅ ⋅ STVpU μ n μ ddddd nBBAA = + + ⋅ ⋅ ⋅ U μ n μ ddd nBBAA J ', J nVS n U μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ =

版权所有:华东理工大学物理化学教研室 17 3) The wider significance of the chemical potential 7.1 Partial molar quantities When the composition changes, not only does μJ show how G changes, it also shows how U, H, and A change too (but under a different set of conditions). The relations of μJ with G, U, H, A are as followings: J nVS ', J n U μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J nTp ', J nG μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J npS ', J n H μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J nVT ', J nA μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ =
版权所有:华东理工大学物理化学教研室 17 3) The wider significance of the chemical potential 7.1 Partial molar quantities When the composition changes, not only does μJ show how G changes, it also shows how U, H, and A change too (but under a different set of conditions). The relations of μJ with G, U, H, A are as followings: J nVS ', J n U μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J nTp ', J nG μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J npS ', J n H μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J nVT ', J nA μ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ =
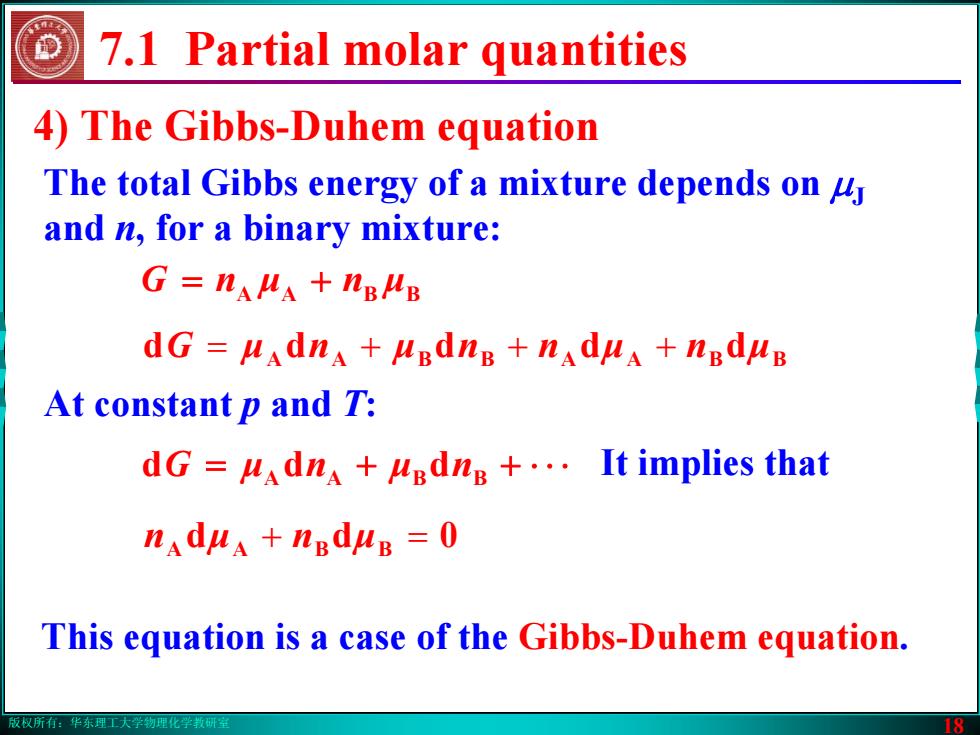
版权所有:华东理工大学物理化学教研室 18 4) The Gibbs-Duhem equation The total Gibbs energy of a mixture depends on μJ and n, for a binary mixture: nG μ n μBBAA 7.1 Partial molar quantities = + G = μ n + μ + nn μ + n ddddd μBBAABBAA At constant p and T: = + + ⋅ ⋅⋅ It implies that G μ n μ ddd nBBAA n μ + n μBBAA = 0dd This equation is a case of the Gibbs-Duhem equation
版权所有:华东理工大学物理化学教研室 18 4) The Gibbs-Duhem equation The total Gibbs energy of a mixture depends on μJ and n, for a binary mixture: nG μ n μBBAA 7.1 Partial molar quantities = + G = μ n + μ + nn μ + n ddddd μBBAABBAA At constant p and T: = + + ⋅ ⋅⋅ It implies that G μ n μ ddd nBBAA n μ + n μBBAA = 0dd This equation is a case of the Gibbs-Duhem equation

版权所有:华东理工大学物理化学教研室 19 4) The Gibbs-Duhem equation 7.1 Partial molar quantities ∑ J = 0d J n μJ The significance of the Gibbs-Duhem equation is that the chemical potential of one component of a mixture cannot change independently of the chemical potentials of the other components. ∑ J = 0d J In general Xn J
版权所有:华东理工大学物理化学教研室 19 4) The Gibbs-Duhem equation 7.1 Partial molar quantities ∑ J = 0d J n μJ The significance of the Gibbs-Duhem equation is that the chemical potential of one component of a mixture cannot change independently of the chemical potentials of the other components. ∑ J = 0d J In general Xn J

版权所有:华东理工大学物理化学教研室 20 4) The Gibbs-Duhem equation 7.1 Partial molar quantities A A A d B dμ n n μ −= In a binary mixture, if one partial molar quantity increases, the other must decrease: This is true for all partial molar quantities
版权所有:华东理工大学物理化学教研室 20 4) The Gibbs-Duhem equation 7.1 Partial molar quantities A A A d B dμ n n μ −= In a binary mixture, if one partial molar quantity increases, the other must decrease: This is true for all partial molar quantities