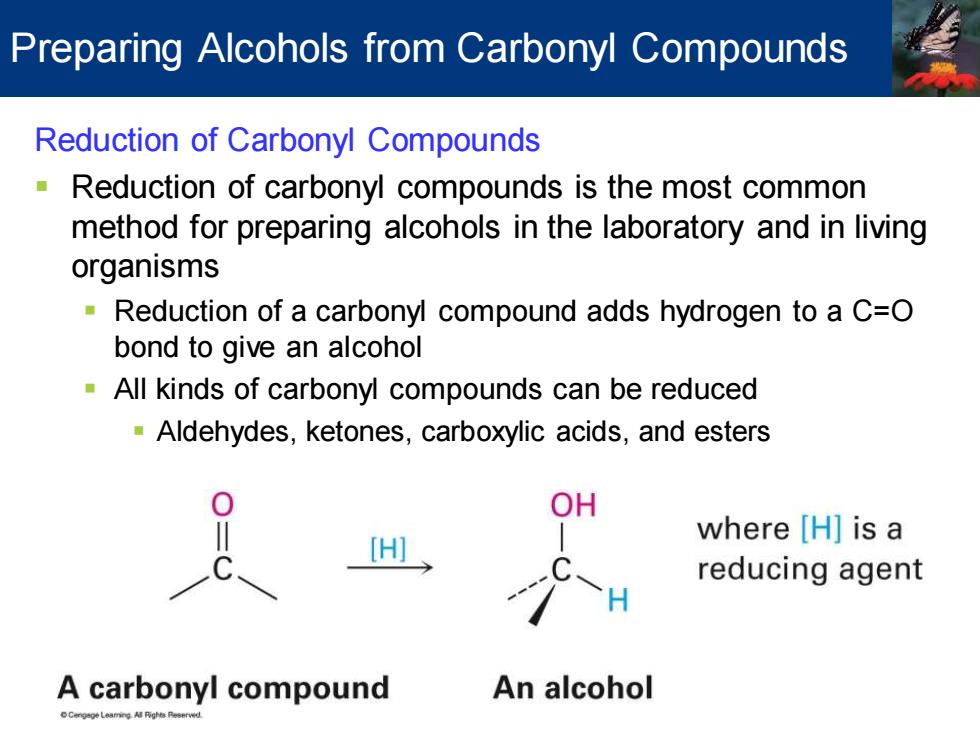
Preparing Alcohols from Carbonyl Compounds Reduction of Carbonyl Compounds Reduction of carbonyl compounds is the most common method for preparing alcohols in the laboratory and in living organisms Reduction of a carbonyl compound adds hydrogen to a C=O bond to give an alcohol All kinds of carbonyl compounds can be reduced Aldehydes,ketones,carboxylic acids,and esters DH where [H]is a reducing agent A carbonyl compound An alcohol
Reduction of Carbonyl Compounds ▪ Reduction of carbonyl compounds is the most common method for preparing alcohols in the laboratory and in living organisms ▪ Reduction of a carbonyl compound adds hydrogen to a C=O bond to give an alcohol ▪ All kinds of carbonyl compounds can be reduced ▪ Aldehydes, ketones, carboxylic acids, and esters Preparing Alcohols from Carbonyl Compounds
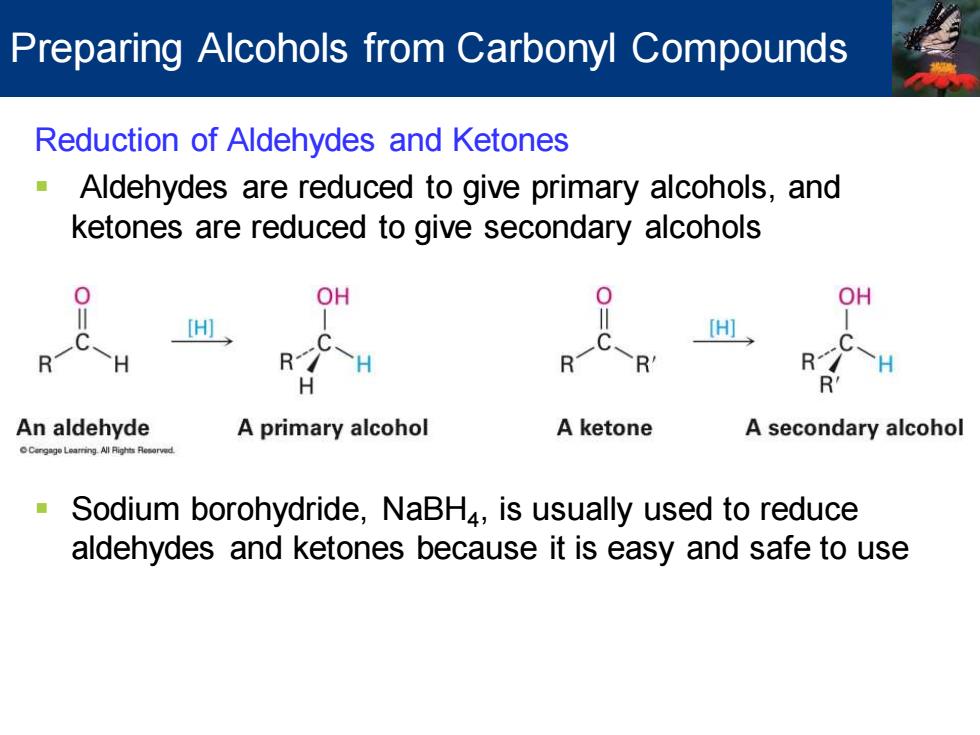
Preparing Alcohols from Carbonyl Compounds Reduction of Aldehydes and Ketones Aldehydes are reduced to give primary alcohols,and ketones are reduced to give secondary alcohols OH OH [H] H R R H R R H R' An aldehyde A primary alcohol A ketone A secondary alcohol Cengage Learring Al Rights Resorved Sodium borohydride,NaBH4,is usually used to reduce aldehydes and ketones because it is easy and safe to use
Reduction of Aldehydes and Ketones ▪ Aldehydes are reduced to give primary alcohols, and ketones are reduced to give secondary alcohols ▪ Sodium borohydride, NaBH4 , is usually used to reduce aldehydes and ketones because it is easy and safe to use Preparing Alcohols from Carbonyl Compounds
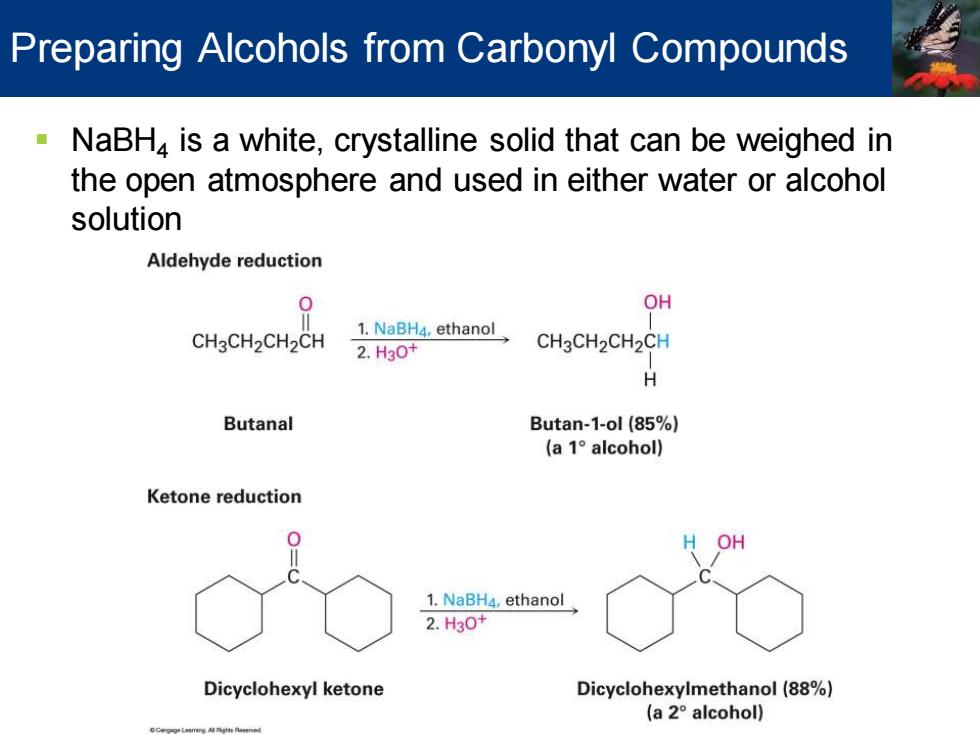
Preparing Alcohols from Carbonyl Compounds NaBH,is a white,crystalline solid that can be weighed in the open atmosphere and used in either water or alcohol solution Aldehyde reduction 0 OH CH3CH2CH2CH 1.NaBH4,ethanol 2.H30+ CH3CH2CH2CH H Butanal Butan-1-ol (85%) (a1°alcohol) Ketone reduction OH 1.NaBH4,ethanol 2.H30+ Dicyclohexyl ketone Dicyclohexylmethanol(88%) (a2°alcohol)
▪ NaBH4 is a white, crystalline solid that can be weighed in the open atmosphere and used in either water or alcohol solution Preparing Alcohols from Carbonyl Compounds
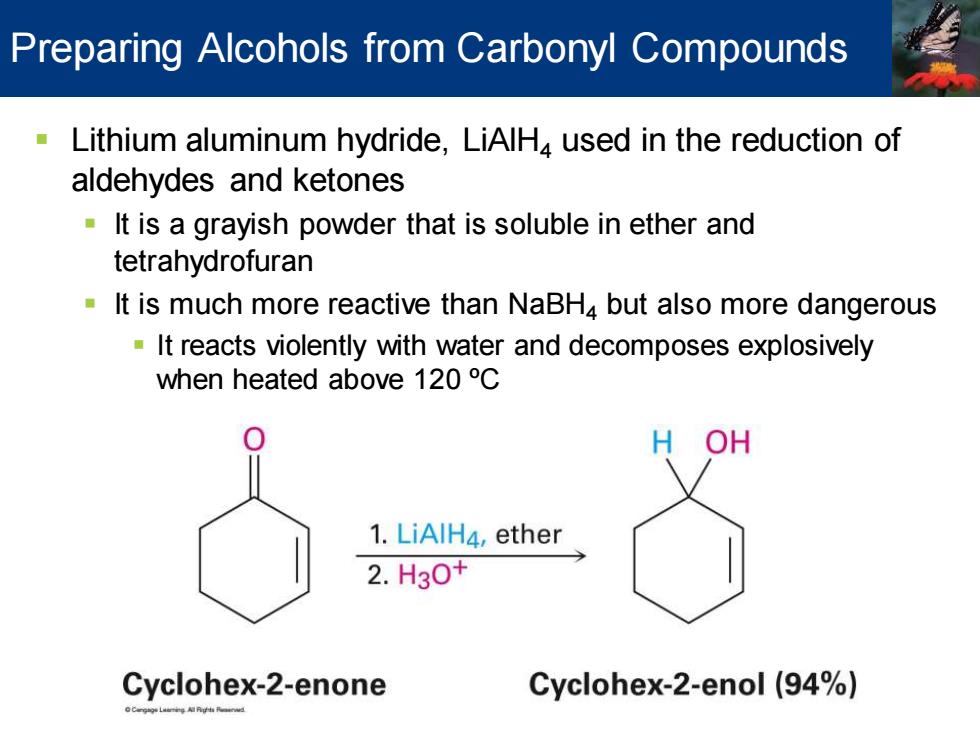
Preparing Alcohols from Carbonyl Compounds Lithium aluminum hydride,LiAlH,used in the reduction of aldehydes and ketones It is a grayish powder that is soluble in ether and tetrahydrofuran It is much more reactive than NaBH4 but also more dangerous It reacts violently with water and decomposes explosively when heated above 120C OH 1.LiAlH4,ether 2.H3O+ Cyclohex-2-enone Cyclohex-2-enol (94%)
▪ Lithium aluminum hydride, LiAlH4 used in the reduction of aldehydes and ketones ▪ It is a grayish powder that is soluble in ether and tetrahydrofuran ▪ It is much more reactive than NaBH4 but also more dangerous ▪ It reacts violently with water and decomposes explosively when heated above 120 ºC Preparing Alcohols from Carbonyl Compounds
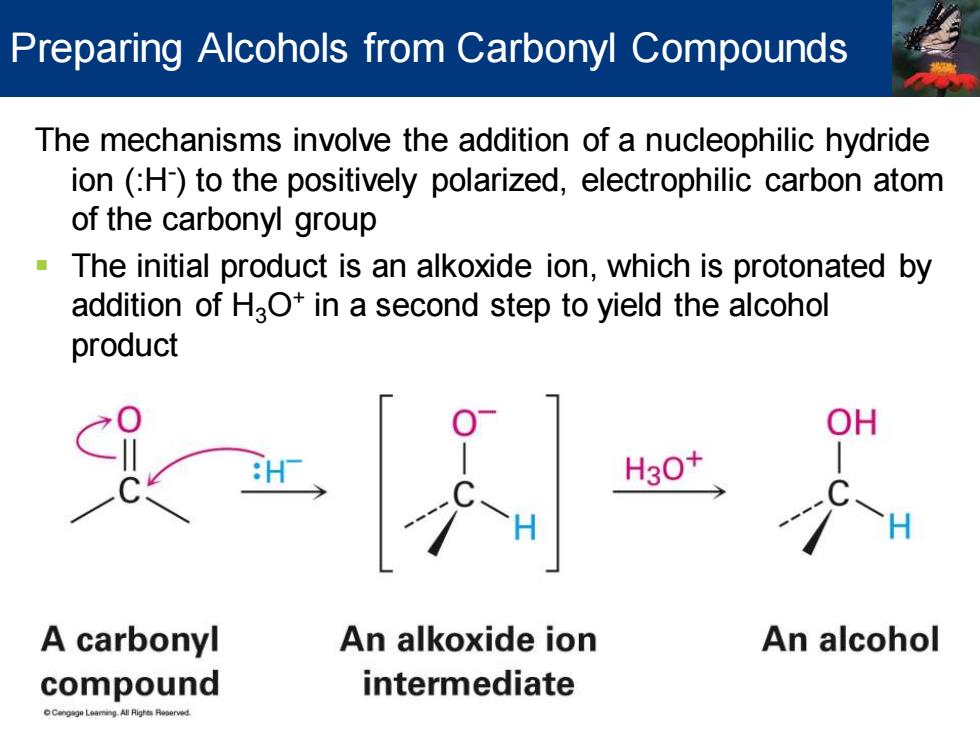
Preparing Alcohols from Carbonyl Compounds The mechanisms involve the addition of a nucleophilic hydride ion (H)to the positively polarized,electrophilic carbon atom of the carbonyl group The initial product is an alkoxide ion,which is protonated by addition of HaO+in a second step to yield the alcohol product A carbonyl An alkoxide ion An alcohol compound intermediate
The mechanisms involve the addition of a nucleophilic hydride ion (:H- ) to the positively polarized, electrophilic carbon atom of the carbonyl group ▪ The initial product is an alkoxide ion, which is protonated by addition of H3O+ in a second step to yield the alcohol product Preparing Alcohols from Carbonyl Compounds