
Lecture 2 Energy and Energy Transfer
Energy and Energy Transfer Lecture 2
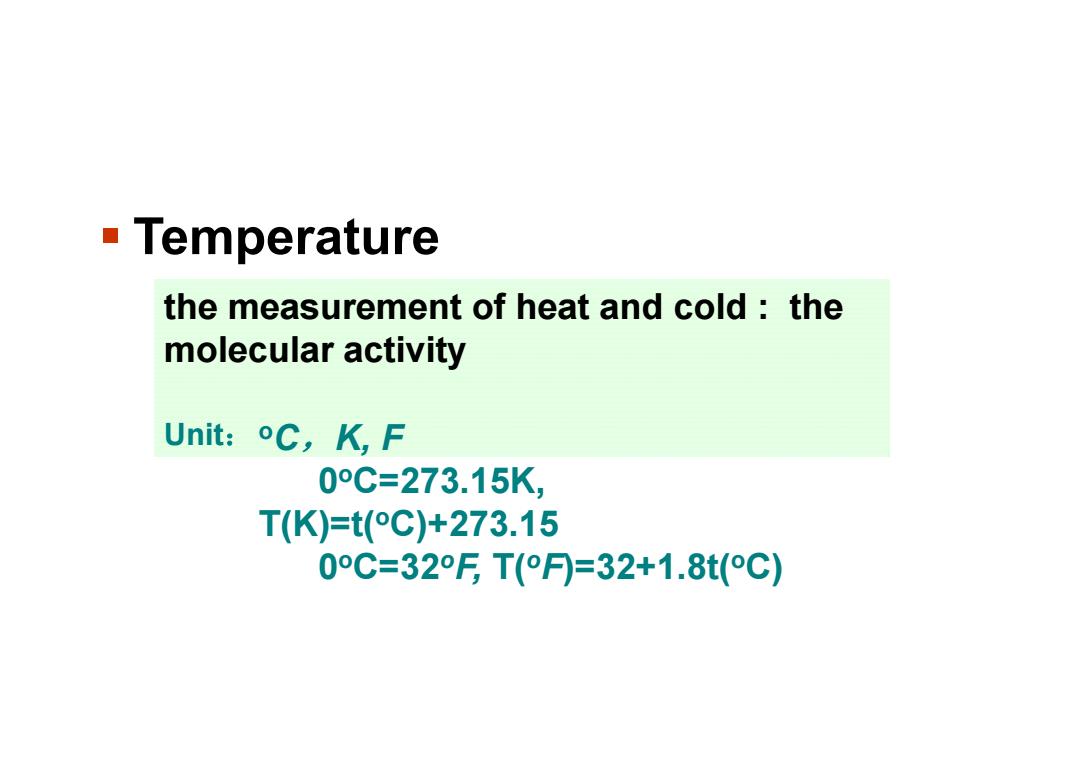
Temperature the measurement of heat and cold:the molecular activity Unit:oC,K,F 0°C=273.15K, T(K)=t(°C)+273.15 0°C=32FT(°月=32+1.8t(°C)
Temperature the measurement of heat and cold : the molecular activity Unit:oC,K, F 0oC=273.15K, T(K)=t(oC)+273.15 0oC=32oF, T(oF)=32+1.8t(oC)

Heat:Energy transferred solely due to temperature difference,Q
Heat: Energy transferred solely due to temperature difference, Q
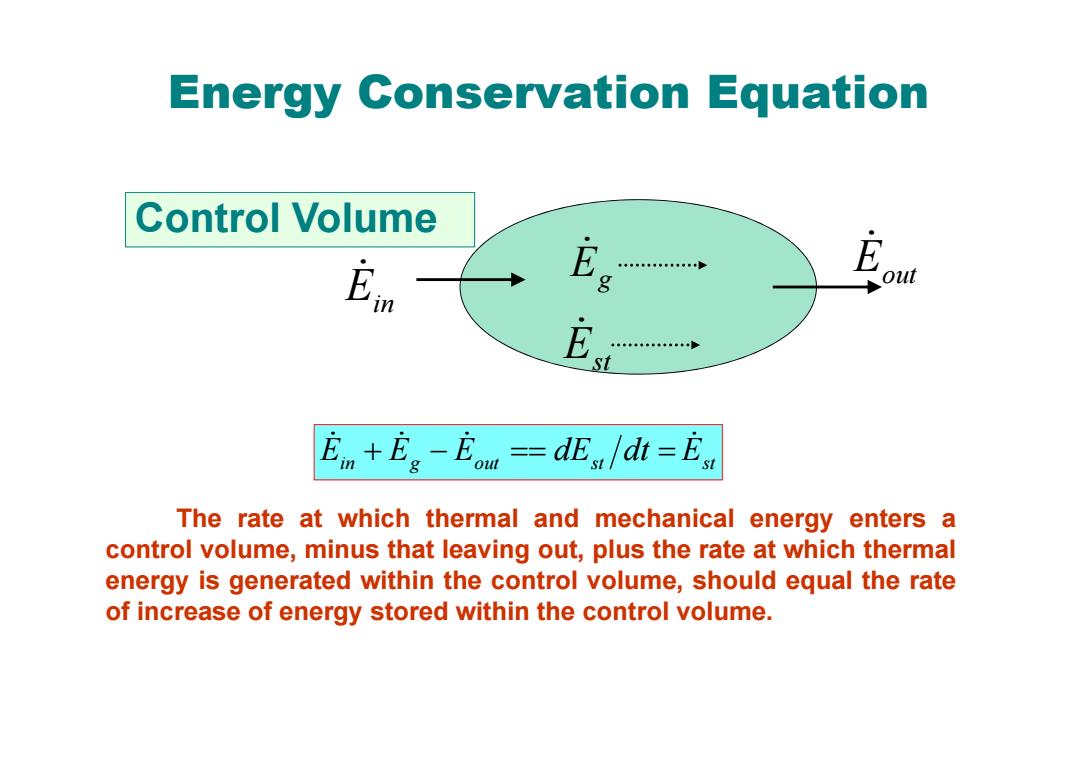
Energy Conservation Equation Control Volume 中年·”年… g out E t En+Eg-Eou =dEs /dt =Est The rate at which thermal and mechanical energy enters a control volume,minus that leaving out,plus the rate at which thermal energy is generated within the control volume,should equal the rate of increase of energy stored within the control volume
Energy Conservation Equation in g out st Est E E E dE dt The rate at which thermal and mechanical energy enters a control volume, minus that leaving out, plus the rate at which thermal energy is generated within the control volume, should equal the rate of increase of energy stored within the control volume. Control Volume Ein Eout E g Est
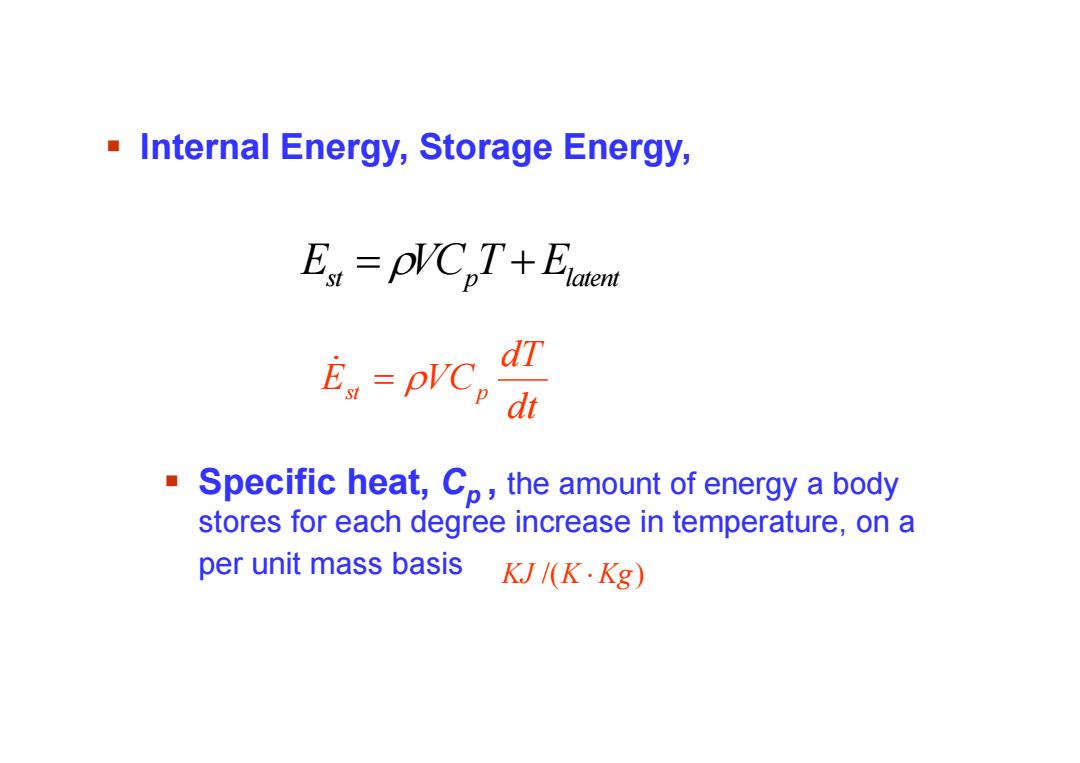
-Internal Energy,Storage Energy, E pVCpT+Elaten dT E=pr℃ dt ■ Specific heat,Co,the amount of energy a body stores for each degree increase in temperature,on a per unit mass basis KJ/(K.Kg)
Internal Energy, Storage Energy, dt dT Est VCp Specific heat, Cp , the amount of energy a body stores for each degree increase in temperature, on a per unit mass basis KJ /(K Kg) E VC T E st p latent