CH3 Hec-si-CHa Tetramethylsilane CH3 TMS is added to the sample as an internal standard. Since silicon is less electronegative than carbon,TMS protons are highly shielded. The TMS signal is defined as zero. Organic protons absorb downfield (to the left)of the TMS signal. Chapter 13 16
Chapter 13 16 Tetramethylsilane • TMS is added to the sample as an internal standard. • Since silicon is less electronegative than carbon, TMS protons are highly shielded. • The TMS signal is defined as zero. • Organic protons absorb downfield (to the left) of the TMS signal. Si CH3 CH3 CH3 H3C

Chemical Shift Measured in parts per million. Ratio of shift downfield from TMS(Hz) to total spectrometer frequency(Hz). The chemical shift has the same value regardless of the machines(same value for 60,100,or 300 MHz machine). Called the delta scale. Chapter 13 17
Chapter 13 17 Chemical Shift • Measured in parts per million. • Ratio of shift downfield from TMS (Hz) to total spectrometer frequency (Hz). • The chemical shift has the same value regardless of the machines (same value for 60, 100, or 300 MHz machine). • Called the delta scale

Delta Scale chemical shift,ppmδ= shift downfield from TMS(in Hz) spectrometer frequency(in MHz) 600Hz 480Hz360Hz240Hz120Hz 0Hz 60 MHz 1098 76 543.21 0 Ppm 8 ↑TMS 3000Hz2400Hz1800Hz1200Hz 600Hz 0Hz 300 MHz 109 8 7 6 54 3 2 1 0 ppm 8 ↑TMS Copyright2010 Pearson Prentice Hall,Inc. Chapter 13 18
Chapter 13 18 Delta Scale

Solved Problem 1 A 300-MHz spectrometer records a proton that absorbs at a frequency 2130 Hz downfield from TMS. (a)Determine its chemical shift,and express this shift as a magnetic field difference. (b)Predict this proton's chemical shift at 60 MHz.In a 60-MHz spectrometer,how far downfield(in gauss and in hertz)from TMS would this proton absorb? Solution We substitute into the equation (a)The chemical shift is the fraction shift downfield(Hz) 2130H=7.10Ppm spectrometer frequency(MHz)300 MHz The chemical shift of this proton is 57.10.The field shift is 70,459 gauss×(7.10×106)=0.500 gauss (b)The chemical shift is unchanged at 60 MHz:57.10.The field shift is 14,092 gauss×(7.10×106)=0.100 gauss The frequency shift is 60MHz×(7.10×106)=426Hz Chapter 13 19
Chapter 13 19 A 300-MHz spectrometer records a proton that absorbs at a frequency 2130 Hz downfield from TMS. (a) Determine its chemical shift, and express this shift as a magnetic field difference. (b) Predict this proton’s chemical shift at 60 MHz. In a 60-MHz spectrometer, how far downfield (in gauss and in hertz) from TMS would this proton absorb? We substitute into the equation Solved Problem 1 Solution
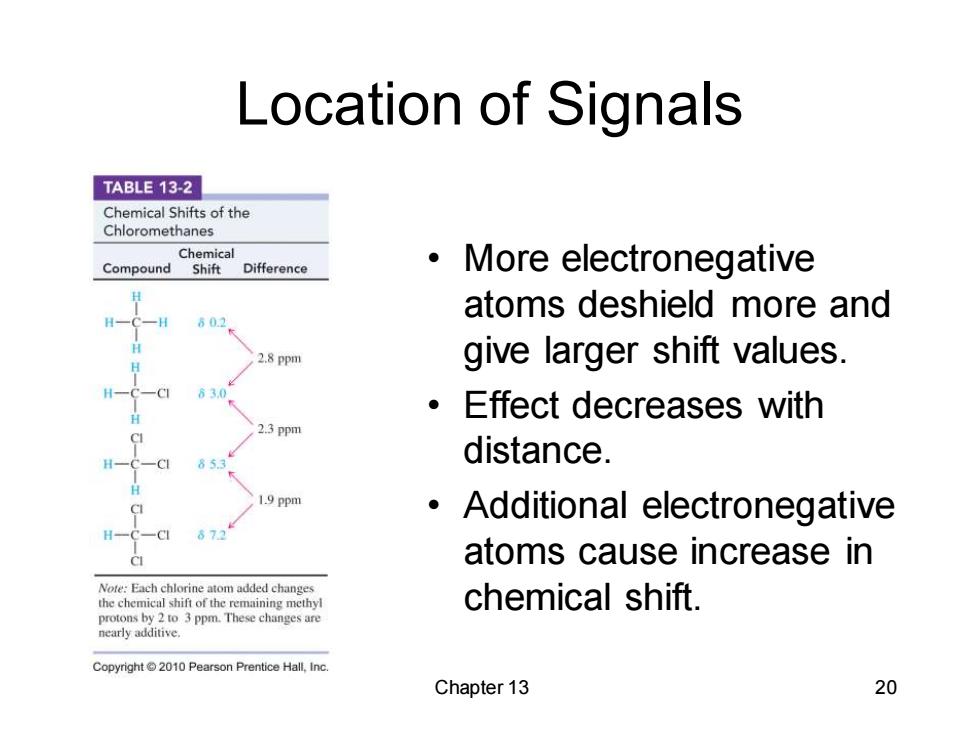
Location of Signals TABLE 13-2 Chemical Shifts of the Chloromethanes Chemical Compound Shift Difference More electronegative 602 atoms deshield more and 2.8 ppm give larger shift values. 63.0 ·Effect decreases with 2.3 ppm 633 distance. 1.9 ppm Additional electronegative 673 atoms cause increase in Note:Each chlorine atom added changes the chemical shift of the remaining methyl chemical shift. protons by 2 to 3 ppm.These changes are nearly additive. Copyright2010 Pearson Prentice Hall,Inc. Chapter 13 20
Chapter 13 20 Location of Signals • More electronegative atoms deshield more and give larger shift values. • Effect decreases with distance. • Additional electronegative atoms cause increase in chemical shift