
24 Bioactive Marine Natural Products Tillek。 1984.23,1331 138.Ochi,M.:Kotsuki.H.:Muraoka.K:Tokoroyama.T.Bull Chem.Soc.(Japan) 1979.52831 139.Ochi,M.:Kotsuki,H.;Inoue,S.:Taniguchi,M.:Tokoroyama,T.Chem.Lent.1979. 831. 140.Moore.R.E.Acc.Chem.Res.1977.10.40. 141. 2.10 D.G.:Gassmann,G.Boland,W.:Marner.F.J.Jaenicke,L.Science 1981 142.M .D.G.:Gassmann.G.:Marner.F.J.:Boland.W.:Jaenicke.L.Science 1982. 2181119 143.Edmonds,J.S.:Francesconi,K.A.Nature 1981.289.602. 144.Edmonds,J.S.;Francesconi,K.A.J.Chem.Soc.Perkin Trans-1 1983,2375. 147 148.Fukuyama.T:Kodama.M.:Miura.L:Kinzyo,Z Kido,M.:Mori.H.:Nakayama Y:Takahashi.M.Chem.Pharm.Bull.1989.37.171. 149.Jormalainen,V.:Honkanen,T.J.Evol.Biol.2004,17.807. 150. 0.Ahn,M.J.:Yoon,K.D.G.:Kim.S.H Kim,N.G. Bull.200 151 Nagayama,K. 152. Glombitza.K.W:Schmidt.A..Nat.Prod.1999.62.1238. 153.Watanabe.K.:Umeda,K.:Miyakado,M.Agr Biol.Chem.1989,53,2513. 154.Ohira,S.;Shirane,F:Nozaki,H.:Yahiro.S.:Nakayama,M.Bull.Chem.Soc. Japan1989,62,2427. Nozaki,H.:Minohara,K.Agr.Biol.Chem.1988,52.3229 156 S.R.:M .Wells,R.J.:Baid-Lambert.J.A.:Jamieson,D.D. 157.Prai.M M.:P ati,F;Rossetti,C.:Berhadini,G.Toxicon 2002,40. 267 158.Burja.A.M.:Abou-Mansour,E.:Banaigs,B.:Payri.C.:Burgess,J.G.J.Micro Methods2002,48,207. 159. Chang.Sitachitta,N.:Rossi.J.V:Roberts.M.A:Flatl.P.M.Jia.J Sherman. Q:Lee.C 2004. M.A.:Gerwick.W.H.Chem.Biol.2004.1.817 162 Tan,L.T.:Sitachitta.N.:Gerwick.W.H.J.Nat.Prod.2003.66.764. 163.Williamson,R.T.;Singh,I.P.:Gerwick.W.H.Tetrahedron 2004.60.7025. I.P:Milligan,K.E.:Gerwick V.H.J.Nat.Prod. 1999,62,1333 166 11n0u 167.Jia Z D.Ier sen.P.R.Fenical.w mMed.Ch .1999.9.2003 168.Berge.J.B:Bourgougnon,N.:Alban,S.Poier.F:Billaudel.S.Chermann.J.C. Robert,J.M.:Franz,G.Planta Med.1999.65.604
24 Bioactive Marine Natural Products 136. Tillekeratne, M. V.; Schimitz, F. J. Phytochem. 1984, 23, 1331. 137. Sun, H. H.; Ferrara, N. M.; McConnell, O. J.; Fenical, W. Tetrahedron Lett. 1980, 21, 3123. 138. Ochi, M.; Kotsuki, H.; Muraoka, K.; Tokoroyama, T. Bull. Chem. Soc. (Japan) 1979, 52, 831. 139. Ochi, M.; Kotsuki, H.; Inoue, S.; Taniguchi, M.; Tokoroyama, T. Chem. Lett. 1979, 831. 140. Moore, R. E. Acc. Chem. Res. 1977, 10, 40. 141. Muller, D. G.; Gassmann, G.; Boland, W.; Marner, F. J.; Jaenicke, L. Science 1981, 212, 1040. 142. Muller, D. G.; Gassmann, G.; Marner, F. J.; Boland, W.; Jaenicke, L. Science 1982, 218, 1119. 143. Edmonds, J. S.; Francesconi, K. A. Nature 1981, 289, 602. 144. Edmonds, J. S.; Francesconi, K. A. J. Chem. Soc. Perkin Trans-I 1983, 2375. 145. Tanaka, J.; Higa, T. Chem. Lett. 1984, 231. 146. De Rosa, S.; De Stefano, S.; Macura, S.; Trivellone, E.; Zavodnik, N. Tetrahedron 1984, 40, 4991. 147. Tringali, C.; Piattelli, M.; Nicolosi, G. Tetrahedron 1984, 40, 799. 148. Fukuyama, T.; Kodama, M.; Miura, I.; Kinzyo, Z.; Kido, M.; Mori, H.; Nakayama, Y.; Takahashi, M. Chem. Pharm. Bull. 1989, 37, 171. 149. Jormalainen, V.; Honkanen, T. J. Evol. Biol. 2004, 17, 807. 150. Ahn, M. J.; Yoon, K. D.; Min, S. Y.; Lee, J. S.; Kim, J. H.; Kim, T. G.; Kim, S. H.; Kim, N. G.; Huh, H.; Kim, J. Biol. Pharm. Bull. 2004, 27, 544. 151. Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. J. Antimicrob. Chemother. 2002, 50, 889. 152. Glombitza, K. W.; Schmidt, A. J. Nat. Prod. 1999, 62, 1238. 153. Watanabe, K.; Umeda, K.; Miyakado, M. Agr. Biol. Chem. 1989, 53, 2513. 154. Ohira, S.; Shirane, F.; Nozaki, H.; Yahiro, S.; Nakayama, M. Bull. Chem. Soc. Japan 1989, 62, 2427. 155. Nozaki, H.; Minohara, K. Agr. Biol. Chem. 1988, 52, 3229. 156. Kazlauskas, R.; Murphy, P.T.; Wells, R. J.; Baid-Lambert, J. A.; Jamieson, D. D. Aust. J. Chem. 1983, 36, 165. 157. Prati, M.; Molteni, M.; Pomati, F.; Rossetti, C.; Berhadini, G. Toxicon 2002, 40, 267. 158. Burja, A. M.; Abou-Mansour, E.; Banaigs, B.; Payri, C.; Burgess, J. G. J. Micro Methods 2002, 48, 207. 159. Chang, Z.; Sitachitta, N.; Rossi, J. V.; Roberts, M. A.; Flatt, P. M.; Jia, J.; Sherman, D. H.; Gerwick, W. H. J. Nat. Prod. 2004, 67, 1356. 160. White, J. D.; Xu, Q.; Lee, C. S.; Valeriote, F. A. Org. Biomol. Chem. 2004, 2, 2092. 161. Edwards, D. J.; Marquez, B. L.; Nogle, L. M.; McPhail, K.; Goeger, D. E.; Roberts, M. A.; Gerwick, W. H. Chem. Biol. 2004, 11, 817. 162. Tan, L. T.; Sitachitta, N.; Gerwick, W. H. J. Nat. Prod. 2003, 66, 764. 163. Williamson, R. T.; Singh, I. P.; Gerwick, W. H. Tetrahedron 2004, 60, 7025. 164. Izumi, A. K.; Moore, R. E. Clin. Dermatol. 1987, 5, 92. 165. Singh, I. P.; Milligan, K. E.; Gerwick, W. H. J. Nat. Prod. 1999, 62, 1333. 166. Yamada, T.; Iwamoto, C.; Yamagoki, N.; Yamanouchi, T.; Minoura, K.; Yamori, T.; Uehara, Y.; Andoh, T.; Umemura, K.; Numata, A. Tetrahedron 2002, 58, 479. 167. Jiang, Z. D.; Jensen, P. R.; Fenical, W. Bioorg. Med. Chem. Lett. 1999, 9, 2003. 168. Berge, J. P.; Bourgougnon, N.; Alban, S.; Pojer, F.; Billaudel, S.; Chermann, J. C.; Robert, J.M.; Franz, G. Planta Med. 1999, 65, 604

bioactive metabolites of marine algae.fungi and bacteria 25 169.Flodin.C.:Whitefield.E D.Phvtochem 2000.5377 170.Huheihel.M.:Ishanu.V.:Tal.J.:Shoshana.A.J.Biochem.Biophry.Methods 2002. 50,189. 171.Osterhage,C.;Konig.G.M.;Holler,U.;Wright,A.D.J.Nat.Prod.2002.65,306 172.Koyanagi,S.:Tanigawa,N.;Nakagawa.H.;Soeda,S.:Shimeno,H.Biochem. H.:Zhang.Y:Li.Z.:Xu.Z.Pha 2004047 N.C 175.O'Brien.A.:Sharp.R.:Russell,N.J.:Roller,S.FEMS Microb.Ecol.2004,48. 157
Bioactive Metabolites of Marine Algae, Fungi and Bacteria 25 169. Flodin, C.; Whitefield, F. D. Phytochem. 2000, 5377. 170. Huheihel, M.; Ishanu, V.; Tal, J.; Shoshana, A. J. Biochem. Biophy. Methods 2002, 50, 189. 171. Osterhage, C.; Konig, G. M.; Holler, U.; Wright, A. D. J. Nat. Prod. 2002, 65, 306. 172. Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Biochem. Pharma. 2003, 65, 173. 173. Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. Pharm. Res. 2004, 50, 47. 174. Teramoto, M.; Rahlert, N.; Misawa, N.; Sandmann, G. FEBS Lett. 2004, 570, 184. 175. O’Brien, A.; Sharp, R.; Russell, N. J.; Roller, S. FEMS Microb. Ecol. 2004, 48, 157

2 Bioactive Metabolites of Marine Invertebrates Te chapcr hteof the marine invertebrtes The ids te noid heterocyclics and nitrogen-sulphur heterocyclics from marine invertebrates have been discussed.The chapter also reviews the bioactive secondary metabolites 1.Introduction Several metabolites of unusual structu have been isolated fron narine ar eof these bioactive metabolite been mainly isolated from marine sponges,jelly fish,sea anemones,corals, bryozoans,molluscs,echinoderms,tunicates and crustaceans. 2.Bioactive Metabolites The bioactive metabolites isolated from marine animals could be divided into steroids,terpenoids,isoprenoids,nonisoprenoids,quinones,brominated compounds,nitrogen heterocyclics,and nitrogen sulphur heterocyclics 2.1 Steroids The bioactive compounds isolated from marine animals,which have steroidal nucleus are insect moulting hormones,sterols and saponins.Karlson22 found
2 Bioactive Metabolites of Marine Invertebrates Abstract The chapter deals with the bioactive metabolites of the marine invertebrates. The chemistry and biological activity of the bioactive steroids, terpenoids, isoprenoid and non-isoprenoid compounds, quinones, brominated compounds, nitrogen heterocyclics and nitrogen-sulphur heterocyclics from marine invertebrates have been discussed. The chapter also reviews the bioactive secondary metabolites isolated in recent past from the marine sponges, jelly fish, sea anemones, corals, bryozoans, molluscs, echinoderms, tunicates and crustaceans. 1. Introduction Several metabolites of unusual structure and exhibiting biological activities have been isolated from marine animals.1-21 Some of these bioactive metabolites have biomedical potential. The bioactive metabolites that are of interest have been mainly isolated from marine sponges, jelly fish, sea anemones, corals, bryozoans, molluscs, echinoderms, tunicates and crustaceans. 2. Bioactive Metabolites The bioactive metabolites isolated from marine animals could be divided into steroids, terpenoids, isoprenoids, nonisoprenoids, quinones, brominated compounds, nitrogen heterocyclics, and nitrogen sulphur heterocyclics. 2.1 Steroids The bioactive compounds isolated from marine animals, which have steroidal nucleus are insect moulting hormones, sterols and saponins. Karlson22 found

Bioactive Metabolites of Marine Invertebrates 27 from crayfish (Jasus lalandei)collected in Australia. Crustecdysone (C27H4407),m.p.240-242C:Amax (EtOH)240 nm (e 12, 670:NMR(pyridine-.ds)81.07H-27).1.20(H-18).1.36(H-26)and(H- 27).1.56(H-21)and 6.17 (H-7),the first crustacean moulting hormone has xygen more in its formula than cdy nd sho wed similar chemical and physical properties.The chemical shift and splitting pattern of two methyl groups in the NMR spectrum of crustecdysone differed markedly from those of the C-18 and C-21 methyl signal in the spectrum of a-ecdysone.This difference was interpreted as generating from the presence one was supporte by the mass spectrum of cruste dysone,which showed th same side chain fragment peak at m/z 99 and 81 as in a-ecdysone but had nuclear fragment peaks at m/z 363 and 345,one mass unit less than the corresponding ion in the mass spectrum of o-ecdysone.This indicated that the side chain Co-C bond cleavage had taken place without hydrogen ent,which (.OH) was expect of vicinal diol.Finally,thes structure 20-Hydroxyecdysone and ecdysone obtained from different sources were shown to have same structure (1,R =OH)on the basis of similar evidences2 30 and later on confirmed to be identical with B-ecdysone.New insect-moulting harmone 2-deoxy-20-dydroxy ecdysone has been isolated in 0016%from oassa The stereochemistry of crustecdysone activity in guinea pig uterus assay. was shown to be the same as that of a-ecdysone.The A/B cis ring fusion was confirmed by the ORD curve,which exhibited a positive cotton effect.32The stereochemistry of the tetracyclic nucleus was established by conversion of B-ecdysone into the known ketone(2).33 Since biological hydroxylation was known to proceed with the retentio of configurati dthonpof for C0()configuration were assigned R in d-ecdysone. was,however,presented by the Grignard reaction on pregnan-20-one.*
Bioactive Metabolites of Marine Invertebrates 27 that an extract of crustacean (Cragon vulgaris) was active in the insect (Calliphora) test. Horn et al23 isolated crustecdysone (1, R = OH)23,24 from crayfish (Jasus lalandei) collected in Australia. Crustecdysone (C27H44O7), m.p. 240-242°C; λmax (EtOH) 240 nm (ε 12, 670); NMR (pyridine-d5) δ1.07 (H-27), 1.20 (H-18), 1.36 (H-26) and (H- 27), 1.56 (H-21) and 6.17 (H-7), the first crustacean moulting hormone has one oxygen more in its molecular formula than α-ecdysone and showed very similar chemical and physical properties. The chemical shift and splitting pattern of two methyl groups in the NMR spectrum of crustecdysone differed markedly from those of the C-18 and C-21 methyl signal in the spectrum of α-ecdysone. This difference was interpreted as generating from the presence of an additional hydroxy group at C-20 in crustecdysone. This assignment was supported by the mass spectrum of crustecdysone, which showed the same side chain fragment peak at m/z 99 and 81 as in α-ecdysone but had nuclear fragment peaks at m/z 363 and 345, one mass unit less than the corresponding ion in the mass spectrum of α-ecdysone. This indicated that the side chain C20-C22 bond cleavage had taken place without hydrogen rearrangement, which was expected of vicinal diol. Finally, the structure (1, R = OH) was assigned to crustecdysone.25 20-Hydroxyecdysone and ecdysone obtained from different sources were shown to have same structure (1, R = OH) on the basis of similar evidences 26-30 and later on confirmed to be identical with β-ecdysone. New insect-moulting harmone 2-deoxy-20-dydroxy ecdysone has been isolated in 0.016% from Zoanthus sp. The compound exhibited promising oxytocic activity in guinea pig uterus assay.31 The stereochemistry of crustecdysone was shown to be the same as that of α-ecdysone. The A/B cis ring fusion was confirmed by the ORD curve, which exhibited a positive cotton effect.32 The stereochemistry of the tetracyclic nucleus was established by conversion of β-ecdysone into the known ketone (2).33 Since biological hydroxylation was known to proceed with the retention of configuration, the configurations at both the positions C-20 and C-22 were assigned R in α-ecdysone.34,35 Chemical proof for C-20 (R) configuration was, however, presented by the Grignard reaction on pregnan-20-one.36,37 1 2 22 23 20
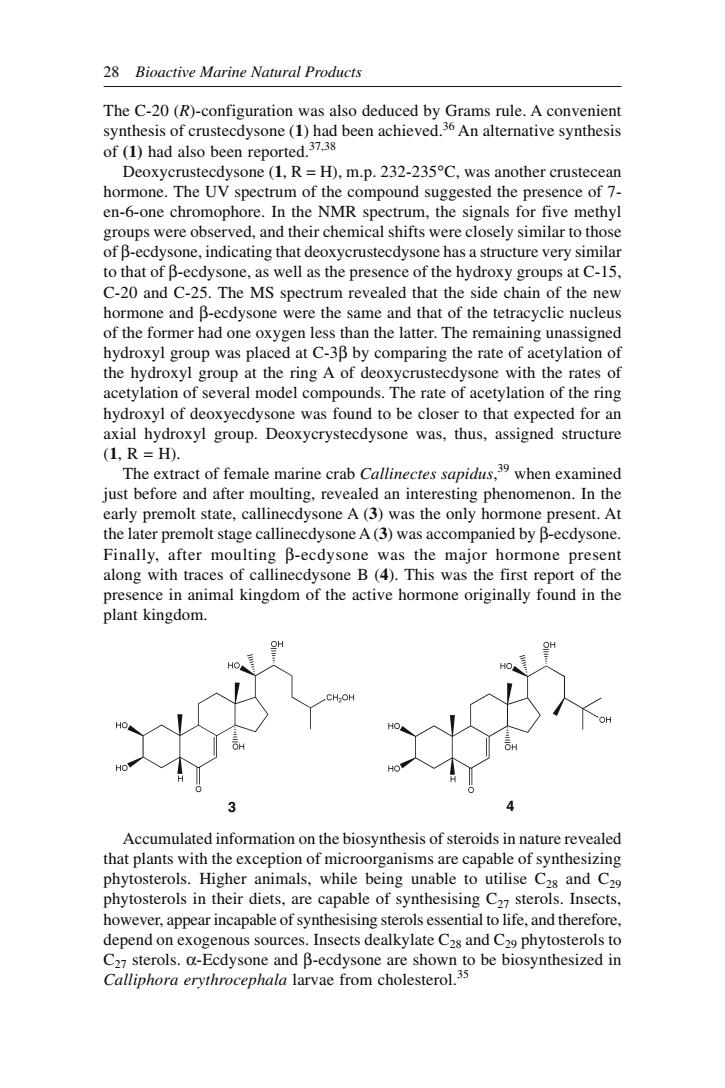
28 Bioactive Marine Natural Products The C-20(R)-configuration was also deduced by Grams rule.A convenient synthesis of crustecdysone(1)had been achieved.36 An alternative synthesis of(1)had also been reported.37.38 ustecdysone(1,R=H),m.p.232-235C,was another crustec hoThe UV spectrm of th compound susted the presenee f en-6-one chromophore.In the NMR spectrum,the signals for five methyl groups were observed,and their chemical shifts were closely similar to those of B-ecdysone,indicating that deoxycrustecdysone has a structure very similar to that of B-ecdysone,as well as the presence of the hydroxy groups at C-15, C-20 and C-25.The MS spectrum revealed that t the side chain of the nev hormone and B-ecdysone were the same and that of the tetracyclic nucleus of the former had one oxygen less than the latter.The remaining unassigned hydroxyl group was placed at C-3B by comparing the rate of acetylation of the hydroxyl group at the ring A of deoxycrustecdysone with the rates of acetylation of several model compounds.The rate of acetylation of the ring hydroxyl of deoxyecdysone was fo und to be closer to that expected for an axial hydroxyl group.Deoxycrystecdysone was,thus,assigned structure 1.R=H). The extract of female marine crab Callinectes sapidus,when examined iust before and after moulting.revealed an interesting phenomenon.In the premol state.cndone A(was the onl hommone present.At er premolt stage callinecdysone A(3)was accompanied by B-ecdysone Finally,after moulting B-ecdysone was the major hormone present along with traces of callinecdysone B(4).This was the first report of the presence in animal kingdom of the active hormone originally found in the olant kingdom. Accumulated information on the biosynthesis of steroids in nature revealed that plants with the exception o roorganisms are capable of synthe phytosterols.Higher animals,while being unable to utilise C2s and C29 phytosterols in their diets,are capable of synthesising C2sterols.Insects, however,appear incapable of synthesising sterols essential to life.and therefore. depend on xogenous sources.Insects dealkylate C2s and C phytosterols to erols.a-Ecdyson and B-ecdysone are Calliphora erythrocephala larvae from cholesterol
28 Bioactive Marine Natural Products The C-20 (R)-configuration was also deduced by Grams rule. A convenient synthesis of crustecdysone (1) had been achieved.36 An alternative synthesis of (1) had also been reported.37,38 Deoxycrustecdysone (1, R = H), m.p. 232-235°C, was another crustecean hormone. The UV spectrum of the compound suggested the presence of 7- en-6-one chromophore. In the NMR spectrum, the signals for five methyl groups were observed, and their chemical shifts were closely similar to those of β-ecdysone, indicating that deoxycrustecdysone has a structure very similar to that of β-ecdysone, as well as the presence of the hydroxy groups at C-15, C-20 and C-25. The MS spectrum revealed that the side chain of the new hormone and β-ecdysone were the same and that of the tetracyclic nucleus of the former had one oxygen less than the latter. The remaining unassigned hydroxyl group was placed at C-3β by comparing the rate of acetylation of the hydroxyl group at the ring A of deoxycrustecdysone with the rates of acetylation of several model compounds. The rate of acetylation of the ring hydroxyl of deoxyecdysone was found to be closer to that expected for an axial hydroxyl group. Deoxycrystecdysone was, thus, assigned structure (1, R = H). The extract of female marine crab Callinectes sapidus, 39 when examined just before and after moulting, revealed an interesting phenomenon. In the early premolt state, callinecdysone A (3) was the only hormone present. At the later premolt stage callinecdysone A (3) was accompanied by β-ecdysone. Finally, after moulting β-ecdysone was the major hormone present along with traces of callinecdysone B (4). This was the first report of the presence in animal kingdom of the active hormone originally found in the plant kingdom. Accumulated information on the biosynthesis of steroids in nature revealed that plants with the exception of microorganisms are capable of synthesizing phytosterols. Higher animals, while being unable to utilise C28 and C29 phytosterols in their diets, are capable of synthesising C27 sterols. Insects, however, appear incapable of synthesising sterols essential to life, and therefore, depend on exogenous sources. Insects dealkylate C28 and C29 phytosterols to C27 sterols. α-Ecdysone and β-ecdysone are shown to be biosynthesized in Calliphora erythrocephala larvae from cholesterol.35 3 4